
What will be compound $A$ in the following reaction?
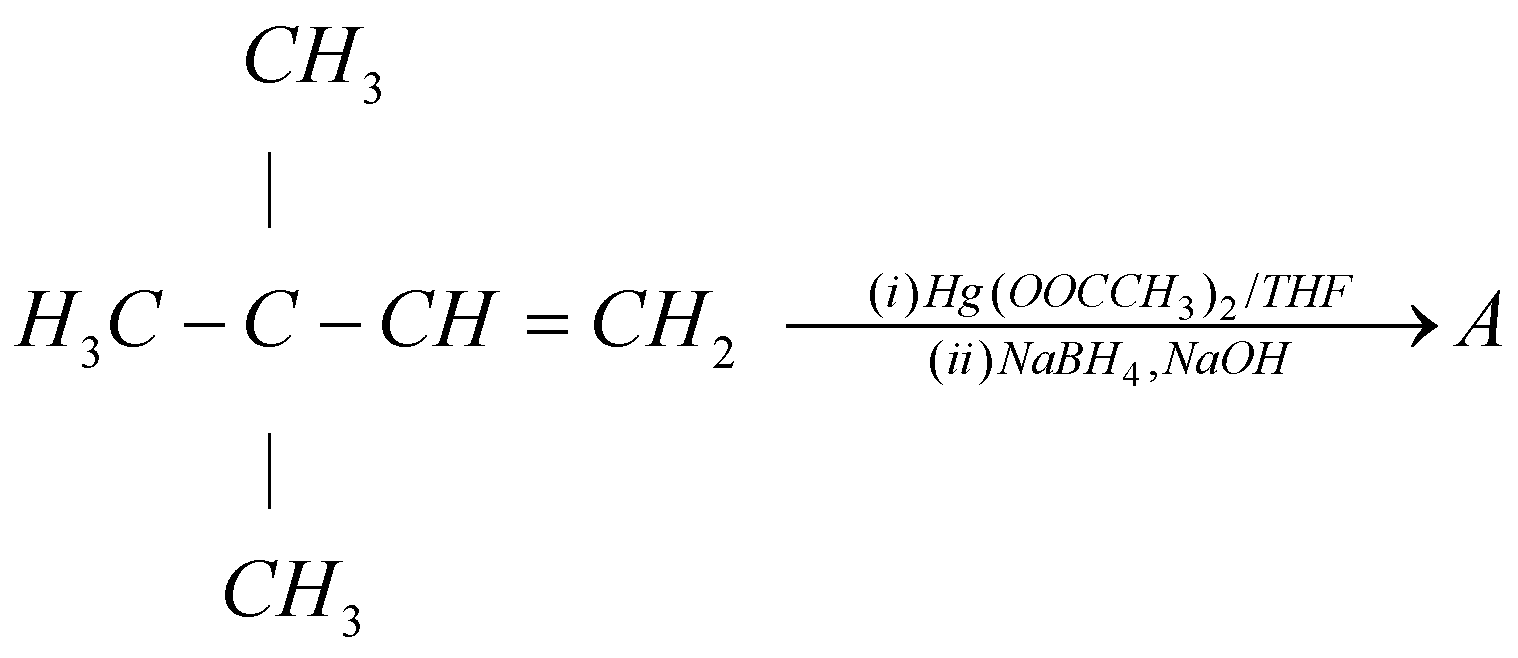
A.)
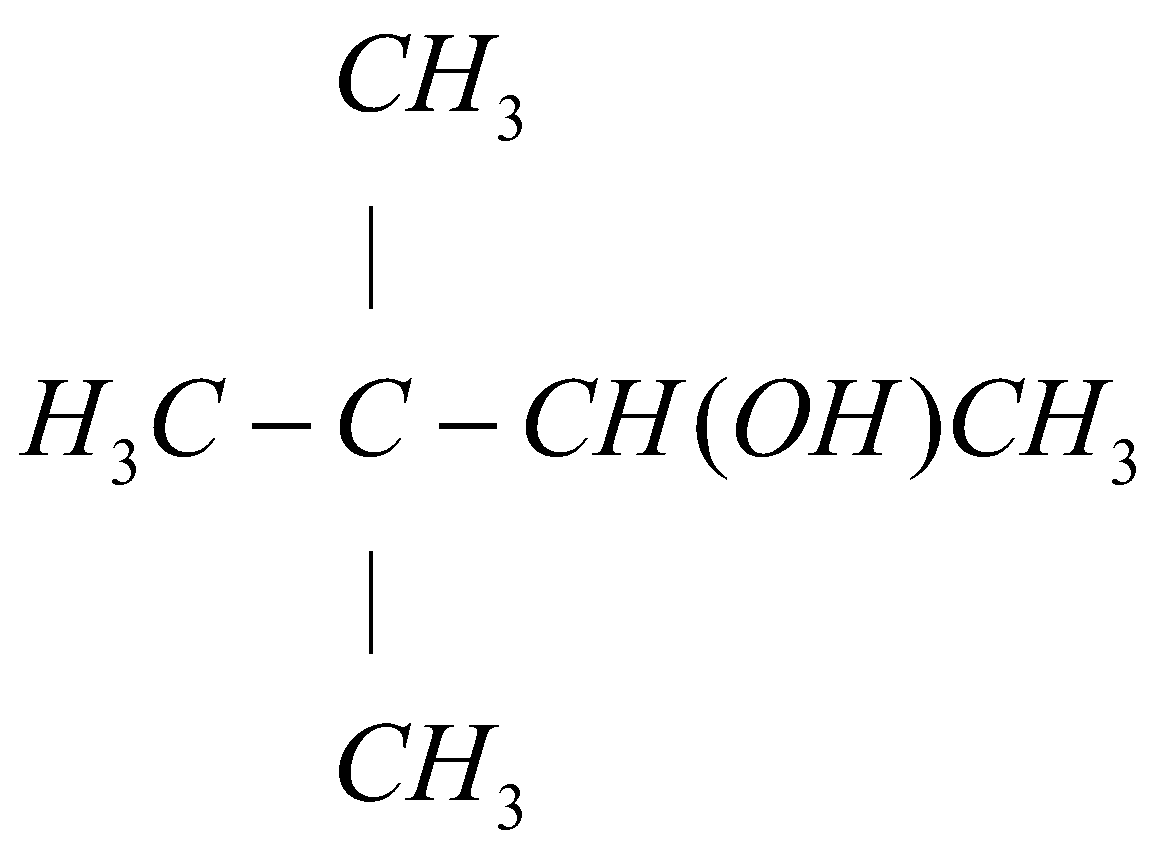
B.)
C.)
D.)
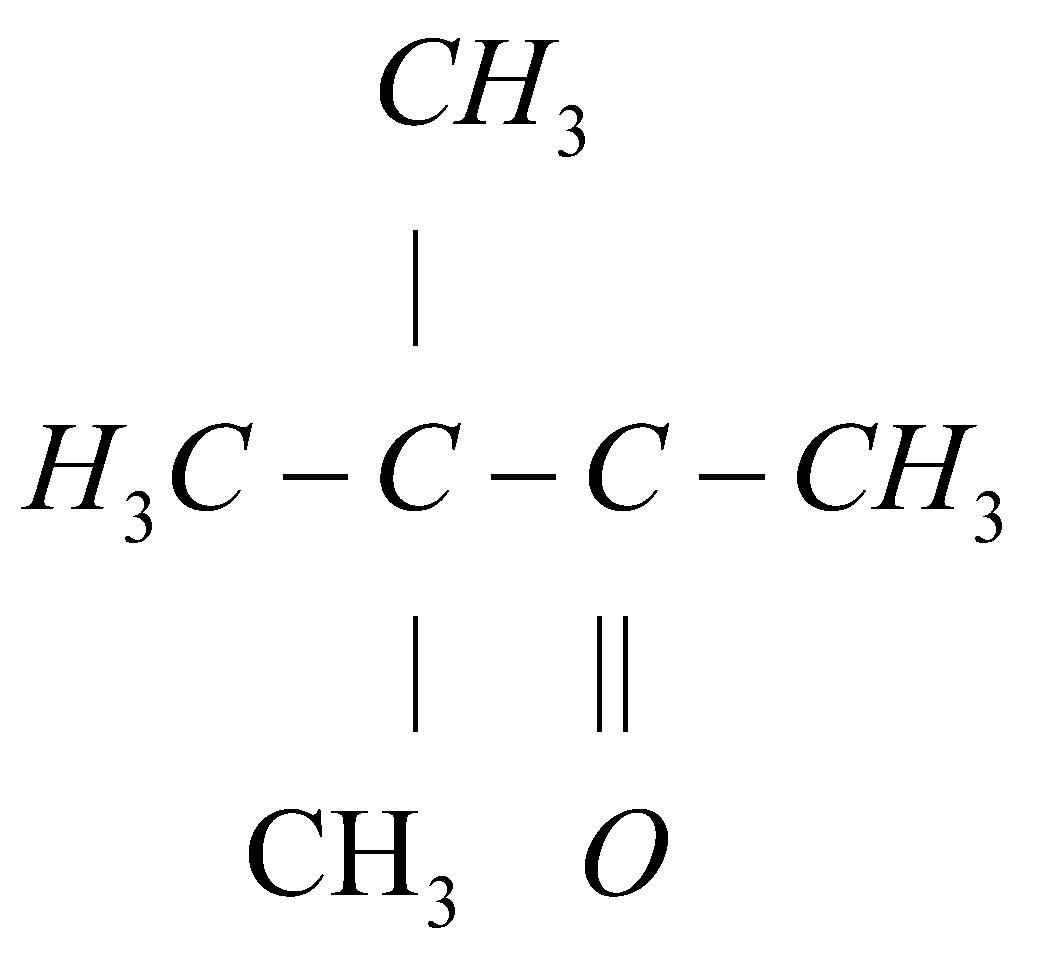
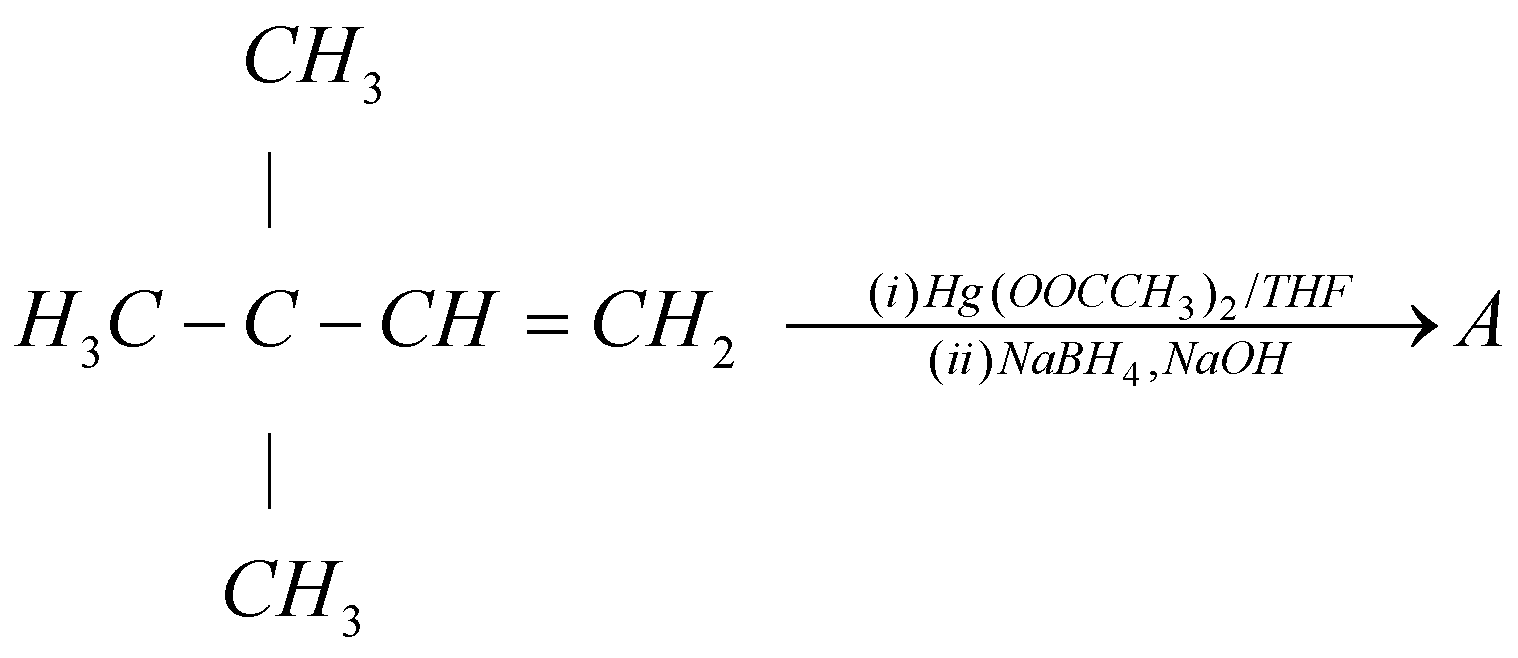
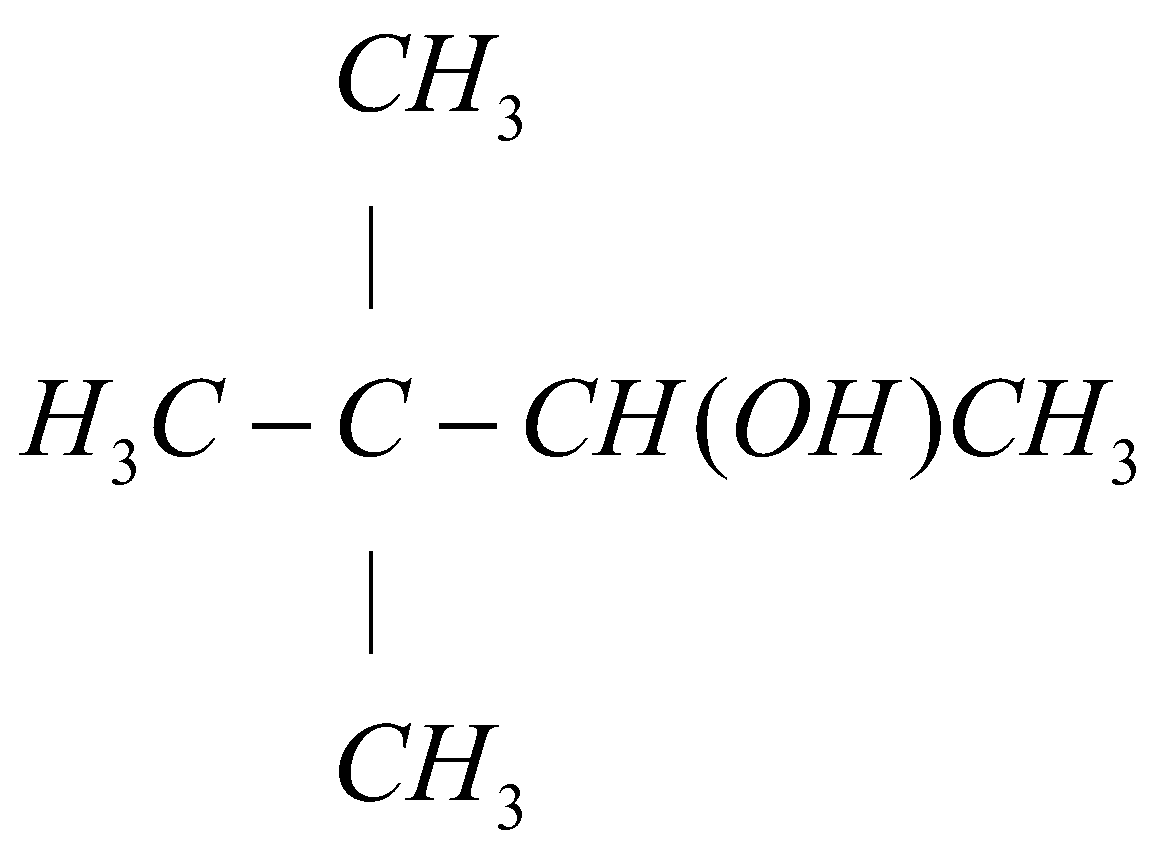

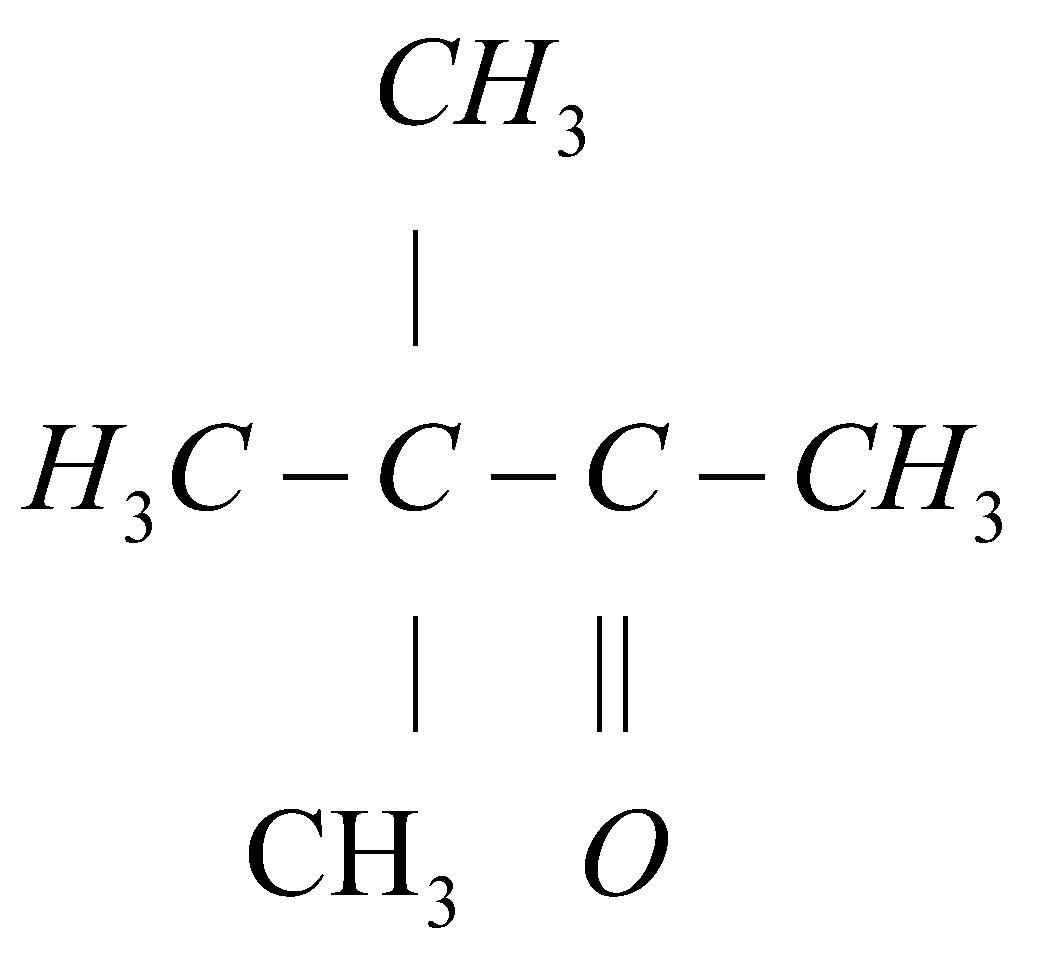
Answer
573.6k+ views
Hint: In this question, this reaction happens by the mechanism of oxymercuration-demercuration mechanism in which the hydroxyl group that is $OH$ attaches to the most substituted carbon and $H$ attaches to the least substituted carbon.
Complete step by step solution:
In this given reaction the regents given that are $Hg(OAc)/THF$ and $NaB{H_4}/NaOH$ are the reagents of oxymercuration-demercuration reaction. Here, in these reagents $Ac$ represents acetone. This oxymercuration- demercuration reaction is a two step pathway that is used to produce corresponding alcohol by the hydration of the alkene. That is by reacting alkene with reagents of oxymercuration- demercuration reaction we can get corresponding alcohol. The oxymercuration- demercuration reaction follows the Markovnikov regioselectivity rule. According to Markovnikov regioselectivity rule, the $OH$ group will attach to the most substituted carbon and the $H$ (Hydrogen) will attach to the least substituted carbon.
In the given alkene,
The carbon number $1$ of alkene functional group is least substituted so the hydrogen ($H$) will attach with the carbon number $1$ and the carbon number $2$ of alkene group is more substituted so hydroxyl group ($OH$) will attach with carbon number $2$. Hence the final reaction can be represented as:
$
{\text{ }}C{H_3}{\text{ }} \\
{\text{ |}} \\
{H_3}C - C - CH = C{H_2}{\text{ }}\xrightarrow[{(ii)NaB{H_4},NaOH}]{{(i)Hg{{(OOCC{H_3})}_2}/THF}} \\
{\text{ |}} \\
{\text{ }}C{H_3} \\
$$
{\text{ }}C{H_3}{\text{ }} \\
{\text{ |}} \\
{H_3}C - C - CH(OH)C{H_3} \\
{\text{ |}} \\
{\text{ }}C{H_3} \\
$
Hence, option (A) is the correct answer.
Note: Always remember that the oxymercuration- demercuration reaction mechanism follows the Markovnikov regioselectivity rule and when alkene reacts with these reagents of oxymercuration demercuration mechanism then the product formed is a corresponding alcohol.
Complete step by step solution:
In this given reaction the regents given that are $Hg(OAc)/THF$ and $NaB{H_4}/NaOH$ are the reagents of oxymercuration-demercuration reaction. Here, in these reagents $Ac$ represents acetone. This oxymercuration- demercuration reaction is a two step pathway that is used to produce corresponding alcohol by the hydration of the alkene. That is by reacting alkene with reagents of oxymercuration- demercuration reaction we can get corresponding alcohol. The oxymercuration- demercuration reaction follows the Markovnikov regioselectivity rule. According to Markovnikov regioselectivity rule, the $OH$ group will attach to the most substituted carbon and the $H$ (Hydrogen) will attach to the least substituted carbon.
In the given alkene,
The carbon number $1$ of alkene functional group is least substituted so the hydrogen ($H$) will attach with the carbon number $1$ and the carbon number $2$ of alkene group is more substituted so hydroxyl group ($OH$) will attach with carbon number $2$. Hence the final reaction can be represented as:
$
{\text{ }}C{H_3}{\text{ }} \\
{\text{ |}} \\
{H_3}C - C - CH = C{H_2}{\text{ }}\xrightarrow[{(ii)NaB{H_4},NaOH}]{{(i)Hg{{(OOCC{H_3})}_2}/THF}} \\
{\text{ |}} \\
{\text{ }}C{H_3} \\
$$
{\text{ }}C{H_3}{\text{ }} \\
{\text{ |}} \\
{H_3}C - C - CH(OH)C{H_3} \\
{\text{ |}} \\
{\text{ }}C{H_3} \\
$
Hence, option (A) is the correct answer.
Note: Always remember that the oxymercuration- demercuration reaction mechanism follows the Markovnikov regioselectivity rule and when alkene reacts with these reagents of oxymercuration demercuration mechanism then the product formed is a corresponding alcohol.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




