
What is compound A.
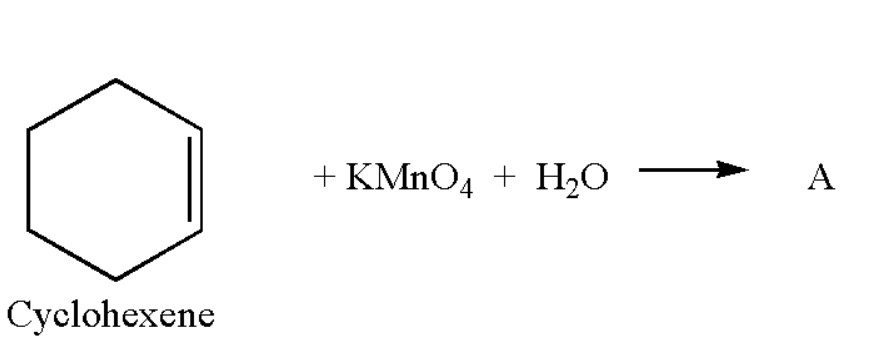
$(i)$ Succinic acid
$(ii)$ Adipic acid
$(iii)$ Oxalic acid
$(iv)$ Malonic acid
Answer
502.5k+ views
Hint: Potassium permanganate is an oxidising agent. When a multiple bond structure reacts with potassium permanganate, the oxidation of the multiple bond takes place. Further when it is used in acidic medium, then addition of ${H^ + }$ takes place with the oxidised product.
Complete answer:
Potassium permanganate is one of the strong oxidising agent which is used to oxidize the given hydrocarbon. For the oxidation of multiple bond structure, potassium permanganate is preferably used. Thus the reaction known as oxidation reaction. The oxidation of Cyclo-hexene takes place in two steps which are as follows:
$(i)$ Potassium permanganate will attack on the double bond of the Cyclo-hexene. Thus it form a complex with the given organic compound. Finally we get the below oxidised product as the main product of the oxidation.
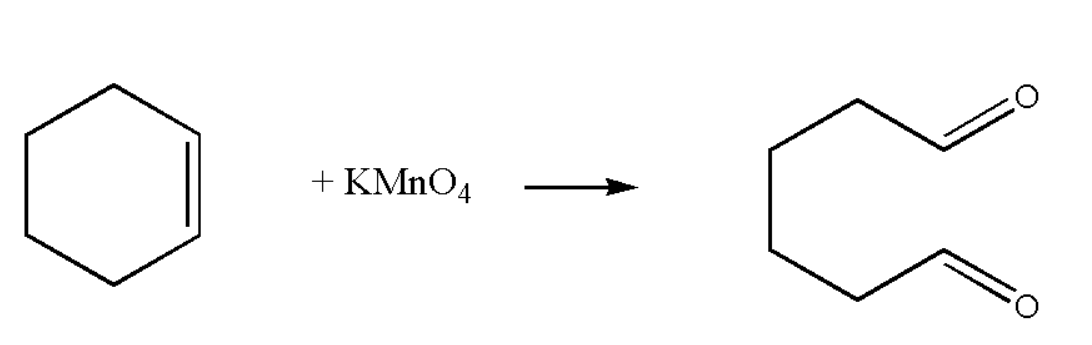
The above product formed is oxidised product since it contain oxygen atoms.
$(ii)$ Now the oxidised product is so formed which will undergoes protonation with the help of water molecule. Therefore when oxidation of the compound takes place in acidic medium then the following reactions takes place.
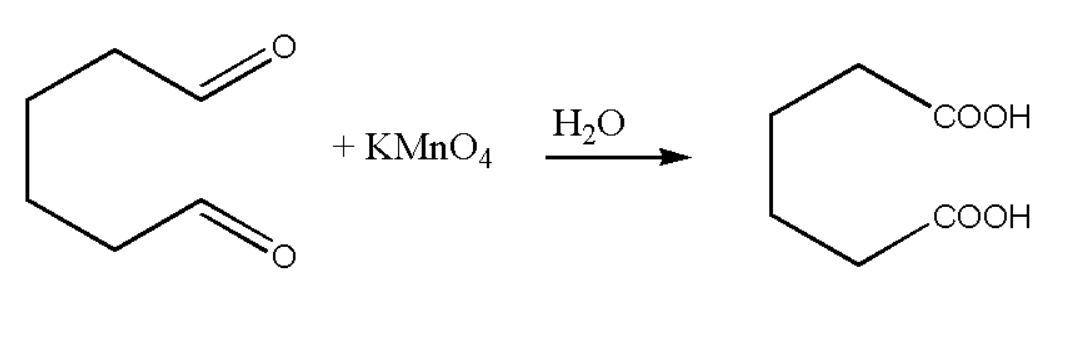
The aldehyde which is formed is again oxidised to carboxylic acid due to excess of potassium permanganate. Thus the final product of the reaction is a adipic acid.
Note:
The aldehyde got converted into carboxylic acid upon oxidation. Also alcohol got converted into aldehyde upon oxidation. The reduction of aldehyde produces alcohol. Potassium permanganate is a strong oxidising agent which can oxidize aldehyde to carboxylic acid. The addition of oxygen to a molecule is also termed as oxidation of a given molecule.
Complete answer:
Potassium permanganate is one of the strong oxidising agent which is used to oxidize the given hydrocarbon. For the oxidation of multiple bond structure, potassium permanganate is preferably used. Thus the reaction known as oxidation reaction. The oxidation of Cyclo-hexene takes place in two steps which are as follows:
$(i)$ Potassium permanganate will attack on the double bond of the Cyclo-hexene. Thus it form a complex with the given organic compound. Finally we get the below oxidised product as the main product of the oxidation.

The above product formed is oxidised product since it contain oxygen atoms.
$(ii)$ Now the oxidised product is so formed which will undergoes protonation with the help of water molecule. Therefore when oxidation of the compound takes place in acidic medium then the following reactions takes place.

The aldehyde which is formed is again oxidised to carboxylic acid due to excess of potassium permanganate. Thus the final product of the reaction is a adipic acid.
Note:
The aldehyde got converted into carboxylic acid upon oxidation. Also alcohol got converted into aldehyde upon oxidation. The reduction of aldehyde produces alcohol. Potassium permanganate is a strong oxidising agent which can oxidize aldehyde to carboxylic acid. The addition of oxygen to a molecule is also termed as oxidation of a given molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




