
What is Compound A?
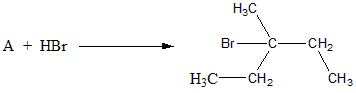
A) $C{H_3}C{H_2}C{H_2}CH = C{H_2}$
B) $C{H_3}C{H_2}CH(C{H_3})CH = C{H_2}$
C) $C{H_3}C{H_2}CH = CHC{H_3}$
D) $C{H_3}C{H_2}C(C{H_3}) = CHC{H_3}$
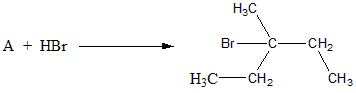
Answer
498.3k+ views
Hint: The given reaction is the addition of HBr in an alkene. The addition should take place in presence of an alkene. Hence compound A should be an alkene.
Complete answer: Markovnikov's rule helps us predict the regioselectivity of the addition of an electrophile in alkenes and alkynes. This rule states that, in Hydrohalogenation of an unsymmetrical alkene, the negative part of the substrate will get attached to that carbon atom having less no. of hydrogen and the positive part of the substrate gets attached to that carbon having more no. of hydrogen.
The product given to us is 3-bromo 3-methyl pentane. To obtain this product via addition of HBr, the reactant should have a double bond between ${C_2}\& {C_3}$. Such a case is fulfilled in Option D).
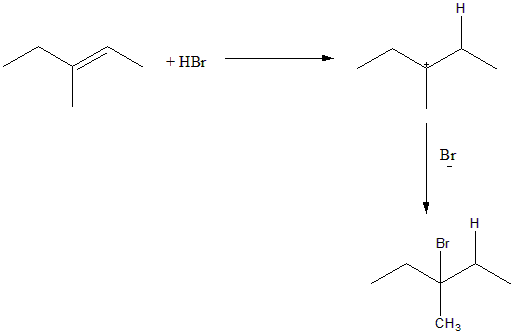
The addition of HBr proceeds via an ionic mechanism and forms a carbocation intermediate. The ${H^ + }$ from HBr will attack on carbon two i.e. ${C_2}$ and form a tertiary carbocation on ${C_3}$ . The Br of the HBr will then attack on the tertiary carbocation to form the desired product. The reaction can be given as:
Hence the correct answer is Option (D).
Note:
If peroxide was added in the reaction, then we would have got 2-bromo,3-methyl pentane which would be an Anti Markovnikov Product. This happens only in the presence of peroxide. The reaction that would occur is a free radical addition reaction. Peroxides from free radicals that initiate the reaction. A strong source of light is required to break the -O-O- peroxide bond, like sunlight, floodlight or UV light.
Complete answer: Markovnikov's rule helps us predict the regioselectivity of the addition of an electrophile in alkenes and alkynes. This rule states that, in Hydrohalogenation of an unsymmetrical alkene, the negative part of the substrate will get attached to that carbon atom having less no. of hydrogen and the positive part of the substrate gets attached to that carbon having more no. of hydrogen.
The product given to us is 3-bromo 3-methyl pentane. To obtain this product via addition of HBr, the reactant should have a double bond between ${C_2}\& {C_3}$. Such a case is fulfilled in Option D).
The addition of HBr proceeds via an ionic mechanism and forms a carbocation intermediate. The ${H^ + }$ from HBr will attack on carbon two i.e. ${C_2}$ and form a tertiary carbocation on ${C_3}$ . The Br of the HBr will then attack on the tertiary carbocation to form the desired product. The reaction can be given as:

Hence the correct answer is Option (D).
Note:
If peroxide was added in the reaction, then we would have got 2-bromo,3-methyl pentane which would be an Anti Markovnikov Product. This happens only in the presence of peroxide. The reaction that would occur is a free radical addition reaction. Peroxides from free radicals that initiate the reaction. A strong source of light is required to break the -O-O- peroxide bond, like sunlight, floodlight or UV light.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE

Explain sex determination in humans with the help of class 12 biology CBSE

India is a sovereign socialist secular democratic republic class 12 social science CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




