
Complete the given reaction.
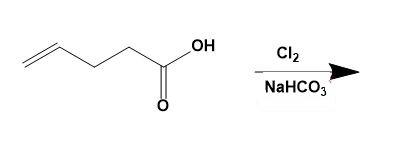
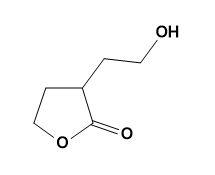
(a)
(b)
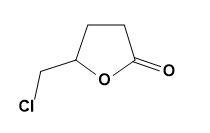
(c)
(d)





Answer
561k+ views
Hint The given reaction is basic carboxylic acid reactions with a halide group and $NaHC{{O}_{3}}$. The reaction taking place to give a specific product can be explained by knowing the basic facts of carboxylic acids and attacking the halide group on it.
Complete step by step solution:
Let us directly see what the reaction gives as a product;
The given compound is 4- pentenoic acid which is then treated with $C{{l}_{2}}$ and $NaHC{{O}_{3}}$ to give 5 - chloromethyl – dihydrofuran – 2 – one. This can be explained by the reaction as,
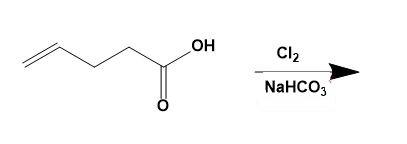
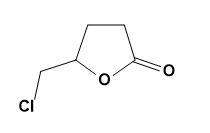
The -OH group present in the carboxylic acid acts as a good nucleophile (due to presence of 2 lone pairs of electrons on oxygen atom) and forms a bond with the carbon; already double bonded to other carbon atoms.
This gives a rise to furan ring formation which then gets stabilised by attaching -Cl and forming the required product. The mechanism can be explained as;
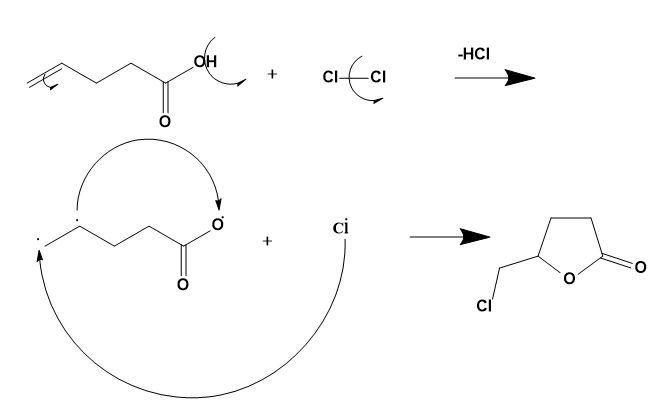
Therefore, option (B) is correct.
Note: Here, we came across the term as furan ring. Furan ring is an aromatic heterocyclic compound having four carbon atoms and an oxygen atom. Whereas, pyran ring is the heterocyclic compound having five carbon atoms with an oxygen atom.
Complete step by step solution:
Let us directly see what the reaction gives as a product;
The given compound is 4- pentenoic acid which is then treated with $C{{l}_{2}}$ and $NaHC{{O}_{3}}$ to give 5 - chloromethyl – dihydrofuran – 2 – one. This can be explained by the reaction as,


The -OH group present in the carboxylic acid acts as a good nucleophile (due to presence of 2 lone pairs of electrons on oxygen atom) and forms a bond with the carbon; already double bonded to other carbon atoms.
This gives a rise to furan ring formation which then gets stabilised by attaching -Cl and forming the required product. The mechanism can be explained as;

Therefore, option (B) is correct.
Note: Here, we came across the term as furan ring. Furan ring is an aromatic heterocyclic compound having four carbon atoms and an oxygen atom. Whereas, pyran ring is the heterocyclic compound having five carbon atoms with an oxygen atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




