
Complete the given nucleophilic substitution reactions, show all electron pairs on both nucleophiles and the leaving group.
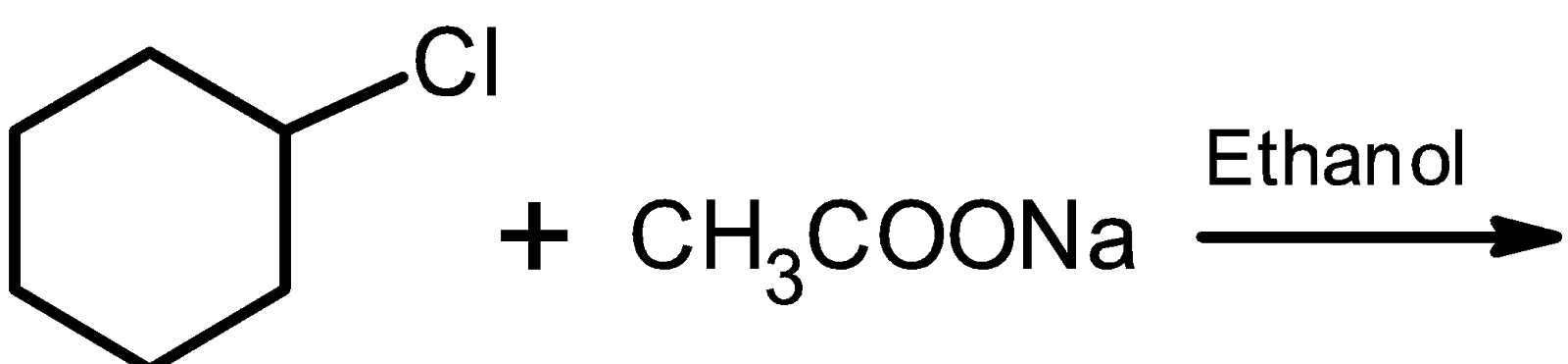
Answer
577.8k+ views
Hint: The substitution of the existing nucleophile by the incoming and more reactive nucleophile is known as the nucleophilic substitution reaction. The acetate ion is a fairly good nucleophile. It pushes out the chloride from the compound and acquires its position. The nucleophilic substitution may proceed through the bimolecular or unimolecular pathways.
Complete answer:
The nucleophiles are the electron-rich species, and have an affinity towards the positive charge. The substitution of one nucleophile by the more reactive nucleophile is the nucleophilic substitution reaction. The nucleophilic substitution reaction follows two types of mechanism:
1) Substitution nucleophilic bimolecular, $\text{ }{{\text{S}}_{\text{N}}}\text{2 }$
2) Substitution nucleophilic unimolecular, $\text{ }{{\text{S}}_{\text{N}}}\text{1 }$
The haloalkanes $\text{ R}-X$ and the nucleophile $\text{ N}{{\text{u}}^{-}}\text{ }$ react with each other such that $\text{ N}{{\text{u}}^{-}}\text{ }$replaces the X. The reaction follows the concerted mechanism through the formation of a transition state. That is bonding breaking and bond formation takes place simultaneously. It is a single-step reaction.
Let's consider the nucleophilic substitution reaction of chlorobenzene with acetate ion.
The nucleophile $\text{ CH3CO}{{\text{O}}^{-}}\text{ }$ (from $\text{ C}{{\text{H}}_{\text{3}}}\text{COONa }$ ) attacks on the partially positive charge carbon atom of the carbon chloride bond from the direction$\text{ 18}{{\text{0}}^{\text{0}}}\text{ }$ away from the chloride atom. That is from the backside.
This leads to a transition state with a partially formed $\text{ C--OOC}{{\text{H}}_{\text{3}}}\text{ }$ bond and partially broken $\text{ C--Cl }$ bond. This process is said to be one step reaction. In this transition state, the negative charge is shared by both the incoming acetate nucleophile as well as outgoing chloride ions. The acetate ion has diminished negative charge because it has partly removed a pair of electrons from the carbon.
The general mechanism is as shown below,
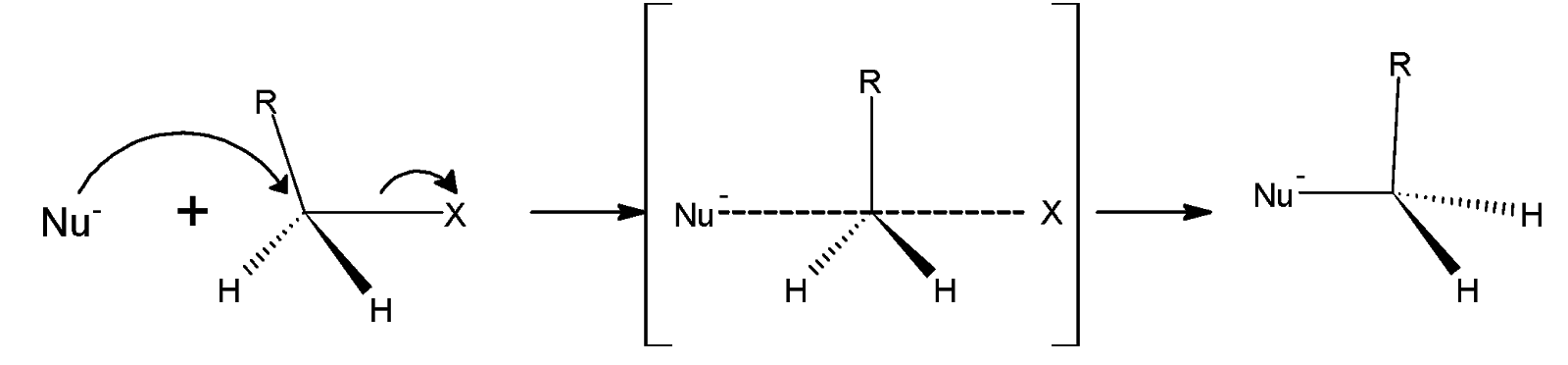
Therefore, both acetate and chloride have a partial negative charge. The remaining three bonds to carbon in the transition state adopt a planar arrangement.
The $\text{ }{{\text{S}}_{\text{N}}}\text{2 }$reaction mechanism of the chlorocyclohexane with the acetate ion in ethanol is as follows,
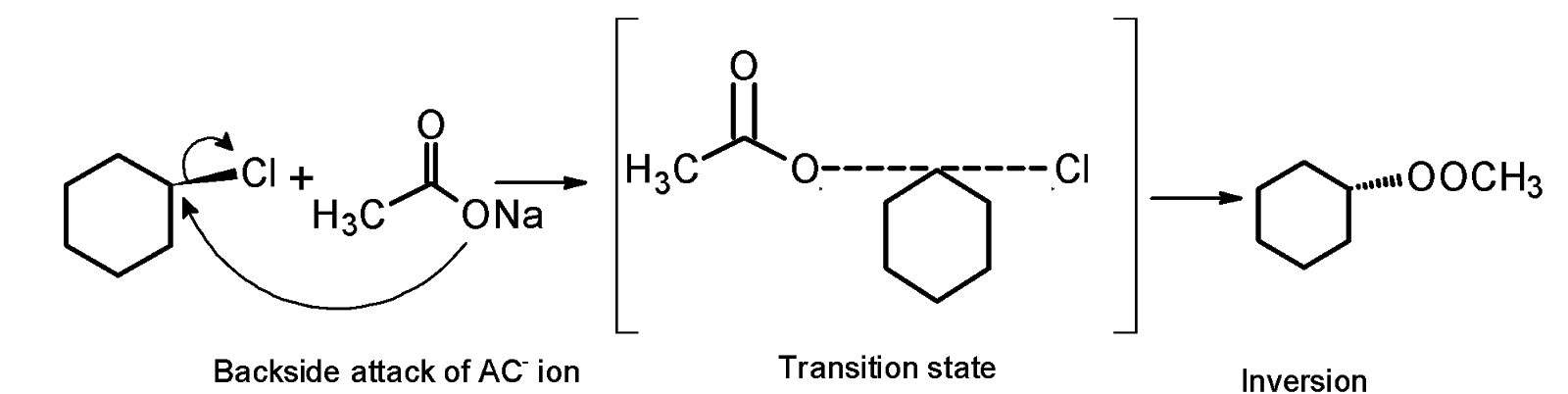
A chloride ion is a leaving group, it takes its electron from the shared covalent bond between chlorine and carbon and leaves as a $\text{ C}{{\text{l}}^{-}}$ .
Note:
The rate of the reaction depends only on the concentration of reactant (substrate) thus it is first-order kinetics. The extent of the reaction of the replacement of the one nucleophile by the other depends on the leaving group ability to knock itself out and the reactivity of the incoming nucleophile. The trend of the order of leaving group ability of halogen is as shown below,
$\text{ }{{\text{I}}^{-}}>\text{ B}{{\text{r}}^{-}}\text{ }>\text{ C}{{\text{l}}^{-}}\text{ }>\text{ }{{\text{F}}^{-}}\text{ }$
Complete answer:
The nucleophiles are the electron-rich species, and have an affinity towards the positive charge. The substitution of one nucleophile by the more reactive nucleophile is the nucleophilic substitution reaction. The nucleophilic substitution reaction follows two types of mechanism:
1) Substitution nucleophilic bimolecular, $\text{ }{{\text{S}}_{\text{N}}}\text{2 }$
2) Substitution nucleophilic unimolecular, $\text{ }{{\text{S}}_{\text{N}}}\text{1 }$
The haloalkanes $\text{ R}-X$ and the nucleophile $\text{ N}{{\text{u}}^{-}}\text{ }$ react with each other such that $\text{ N}{{\text{u}}^{-}}\text{ }$replaces the X. The reaction follows the concerted mechanism through the formation of a transition state. That is bonding breaking and bond formation takes place simultaneously. It is a single-step reaction.
Let's consider the nucleophilic substitution reaction of chlorobenzene with acetate ion.
The nucleophile $\text{ CH3CO}{{\text{O}}^{-}}\text{ }$ (from $\text{ C}{{\text{H}}_{\text{3}}}\text{COONa }$ ) attacks on the partially positive charge carbon atom of the carbon chloride bond from the direction$\text{ 18}{{\text{0}}^{\text{0}}}\text{ }$ away from the chloride atom. That is from the backside.
This leads to a transition state with a partially formed $\text{ C--OOC}{{\text{H}}_{\text{3}}}\text{ }$ bond and partially broken $\text{ C--Cl }$ bond. This process is said to be one step reaction. In this transition state, the negative charge is shared by both the incoming acetate nucleophile as well as outgoing chloride ions. The acetate ion has diminished negative charge because it has partly removed a pair of electrons from the carbon.
The general mechanism is as shown below,

Therefore, both acetate and chloride have a partial negative charge. The remaining three bonds to carbon in the transition state adopt a planar arrangement.
The $\text{ }{{\text{S}}_{\text{N}}}\text{2 }$reaction mechanism of the chlorocyclohexane with the acetate ion in ethanol is as follows,

A chloride ion is a leaving group, it takes its electron from the shared covalent bond between chlorine and carbon and leaves as a $\text{ C}{{\text{l}}^{-}}$ .
Note:
The rate of the reaction depends only on the concentration of reactant (substrate) thus it is first-order kinetics. The extent of the reaction of the replacement of the one nucleophile by the other depends on the leaving group ability to knock itself out and the reactivity of the incoming nucleophile. The trend of the order of leaving group ability of halogen is as shown below,
$\text{ }{{\text{I}}^{-}}>\text{ B}{{\text{r}}^{-}}\text{ }>\text{ C}{{\text{l}}^{-}}\text{ }>\text{ }{{\text{F}}^{-}}\text{ }$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




