
Complete the following reaction-
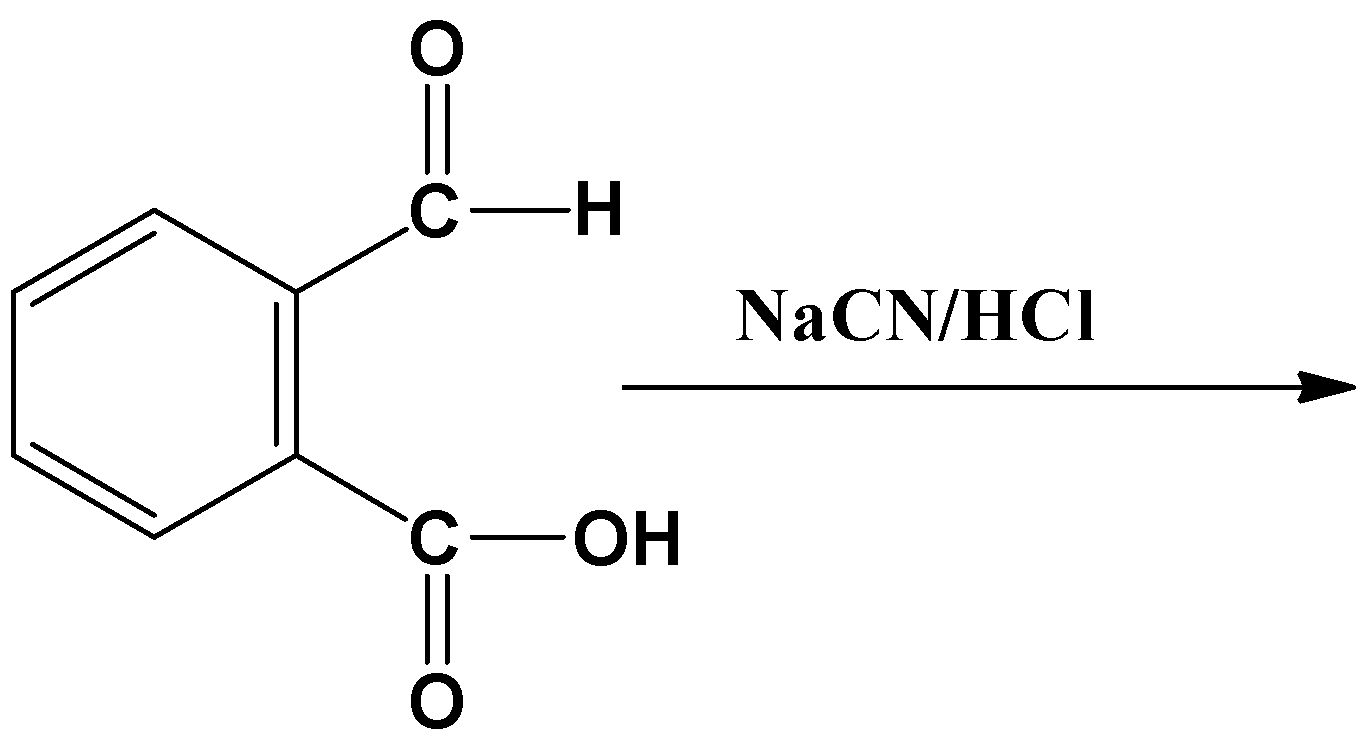

Answer
594.3k+ views
Hint: The attacking reagent is a nucleophile. The reaction will be selective for carbonyl carbon only.
Complete step by step solution:
The reagent given in the above case is a sodium salt in an acidic medium. When hydrochloric acid reacts with sodium cyanide, it produces hydrogen cyanide gas. Therefore the reacting component here is$HCN$. The cyanide ion ($C{{N}^{-}}$) acts as the nucleophile. It is a slow process on its own and often a base is used as a catalyst to increase the overall speed.
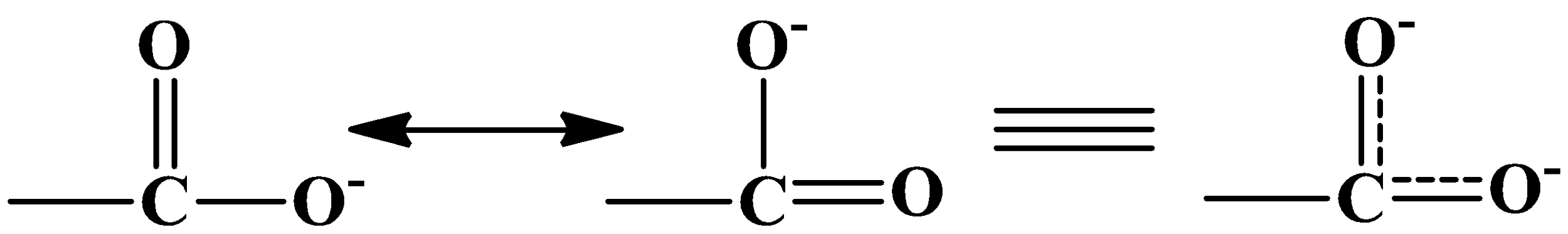
The nucleophile reacts to the carbonyl carbon because it has a partial positive charge on it. This is because of the oxygen atom’s high electronegative nature. In the substrate given above there are two carbonyl carbons, one in acid and another in aldehyde. The reaction will only take place on the carbonyl carbon which has a substantial positive charge, which in this case is the aldehyde. The carboxyl group’s carbon atom also has a positive charge but due to the effect of resonance it becomes less reactive than that of the aldehyde. The resonance is as shown below:
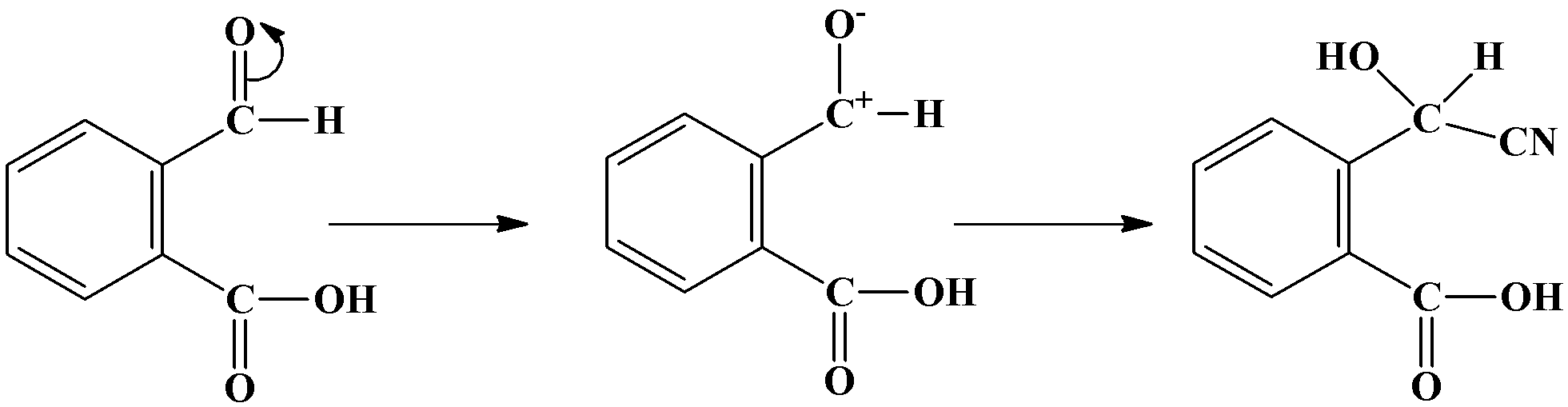
When the cyanide ion ($C{{N}^{-}}$) will attack, the double bond between oxygen and carbon in the carbonyl carbon will break towards the oxygen atom giving oxygen a negative charge and carbon a positive charge. The cyanide ion gets attached to the carbocation and sodium ion links to the oxygen atom. It is as shown below:
Additional Information:
- The products formed above belong to the class of cyanohydrins. They are a useful synthetic intermediate.
- The can be converted back into the original compound if needed.
Note:
- A nucleophilic or electrophilic reaction will only apply to the substituent which proves to be the most competitive. This nature of chemical reactions can be explained if we consider why these reactions occur in the first place. The product is always more stable than the reactants (some exceptions do exist) and therefore the reaction always follows the path of least resistance.
Complete step by step solution:
The reagent given in the above case is a sodium salt in an acidic medium. When hydrochloric acid reacts with sodium cyanide, it produces hydrogen cyanide gas. Therefore the reacting component here is$HCN$. The cyanide ion ($C{{N}^{-}}$) acts as the nucleophile. It is a slow process on its own and often a base is used as a catalyst to increase the overall speed.
The nucleophile reacts to the carbonyl carbon because it has a partial positive charge on it. This is because of the oxygen atom’s high electronegative nature. In the substrate given above there are two carbonyl carbons, one in acid and another in aldehyde. The reaction will only take place on the carbonyl carbon which has a substantial positive charge, which in this case is the aldehyde. The carboxyl group’s carbon atom also has a positive charge but due to the effect of resonance it becomes less reactive than that of the aldehyde. The resonance is as shown below:

When the cyanide ion ($C{{N}^{-}}$) will attack, the double bond between oxygen and carbon in the carbonyl carbon will break towards the oxygen atom giving oxygen a negative charge and carbon a positive charge. The cyanide ion gets attached to the carbocation and sodium ion links to the oxygen atom. It is as shown below:

Additional Information:
- The products formed above belong to the class of cyanohydrins. They are a useful synthetic intermediate.
- The can be converted back into the original compound if needed.
Note:
- A nucleophilic or electrophilic reaction will only apply to the substituent which proves to be the most competitive. This nature of chemical reactions can be explained if we consider why these reactions occur in the first place. The product is always more stable than the reactants (some exceptions do exist) and therefore the reaction always follows the path of least resistance.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




