
Complete the following equation:


Answer
570.9k+ views
Hint:Ethyne is a hydrocarbon with the chemical formula ${C_2}{H_2}$. Ethylene is a colorless gas. Ethylene is widely used in the industry as a fuel. It is also called acetylene. When ethylene gas is passed in a red hot iron tube, it leads to the formation of an aromatic compound.
Complete step by step answer:
Molecules of ethyne gas are treated in a red hot iron tube where it undergoes cyclic polymerization to yield benzene.
Benzene is obtained from ethyne gas via the process of cyclic polymerization. In this process, Ethyne gas is passed through a red hot iron tube at\[873{ }K\]. The ethyne molecule then undergoes cyclic polymerization and forms aromatic compound benzene.
In the process of cyclic polymerization, triple bonds of ethyne are broken and substituted by hydrogen in place of that and then these molecules rearrange themselves into a much more stable structure of benzene in the presence of an organometallic Nickel catalyst.
\[3\] molecules of ethyne gas are reacted to yield one molecule of benzene. The reaction for this is;
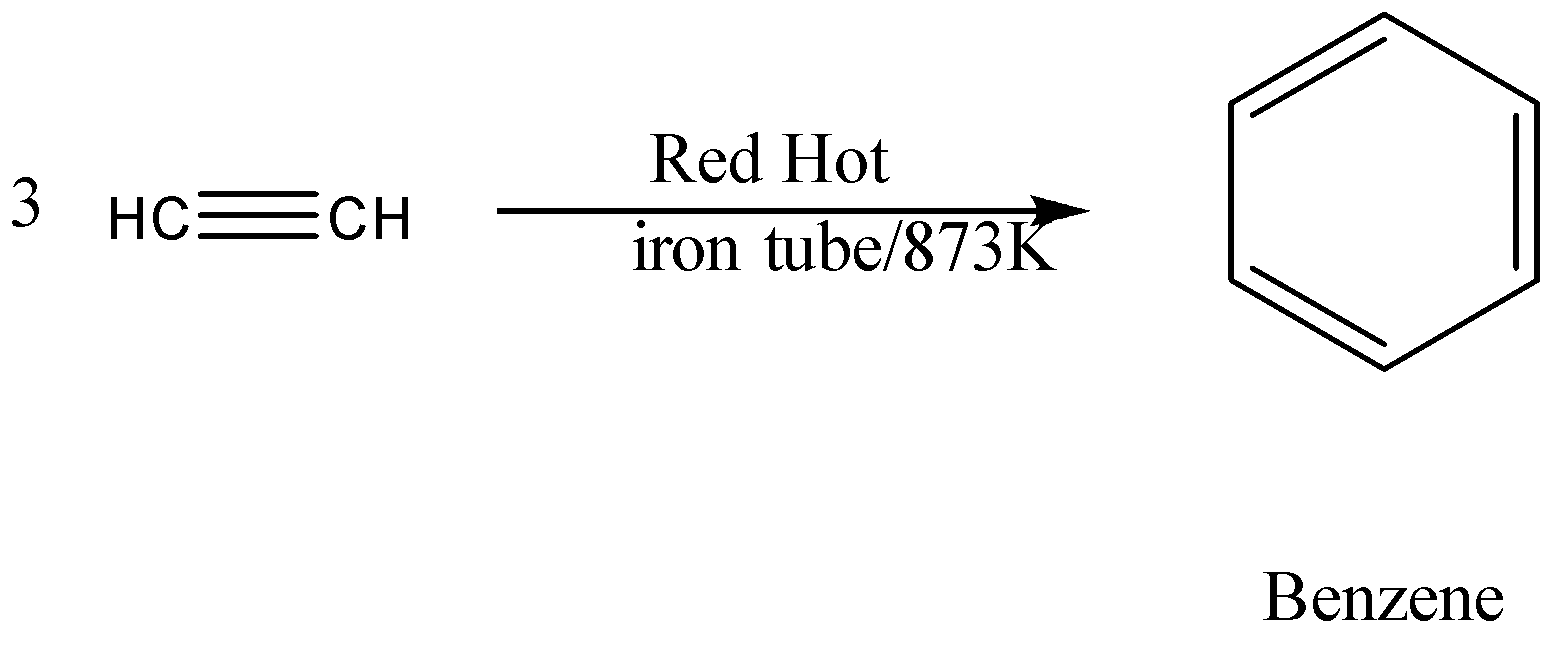
Benzene is an aromatic compound. Its chemical formula is ${C_6}{H_6}$. In benzene, each carbon atom is arranged in a six-membered ring and is bonded to one hydrogen atom. According to molecular orbital, the stability of benzene is due to the formation of $3$ delocalized $\pi - $ orbitals on the six carbon atoms, Thus making benzene a resonance stabilized structure.
Note:
Benzene can also be produced from aromatic acids, phenols, or sulphonic acids using different reaction mechanisms and catalysts. In the industry, it is used in the making of different types of lubricants, rubbers, dyes, detergents, drugs, and pesticides.
Complete step by step answer:
Molecules of ethyne gas are treated in a red hot iron tube where it undergoes cyclic polymerization to yield benzene.
Benzene is obtained from ethyne gas via the process of cyclic polymerization. In this process, Ethyne gas is passed through a red hot iron tube at\[873{ }K\]. The ethyne molecule then undergoes cyclic polymerization and forms aromatic compound benzene.
In the process of cyclic polymerization, triple bonds of ethyne are broken and substituted by hydrogen in place of that and then these molecules rearrange themselves into a much more stable structure of benzene in the presence of an organometallic Nickel catalyst.
\[3\] molecules of ethyne gas are reacted to yield one molecule of benzene. The reaction for this is;

Benzene is an aromatic compound. Its chemical formula is ${C_6}{H_6}$. In benzene, each carbon atom is arranged in a six-membered ring and is bonded to one hydrogen atom. According to molecular orbital, the stability of benzene is due to the formation of $3$ delocalized $\pi - $ orbitals on the six carbon atoms, Thus making benzene a resonance stabilized structure.
Note:
Benzene can also be produced from aromatic acids, phenols, or sulphonic acids using different reaction mechanisms and catalysts. In the industry, it is used in the making of different types of lubricants, rubbers, dyes, detergents, drugs, and pesticides.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




