
: Complete the following equation.
$CH \equiv CH\xrightarrow{{NaN{H_2}}}?\xrightarrow{{C{H_3}Br}}?\xrightarrow{{H{g^{2 + }}/{H^ + }}}?\xrightarrow{{{H^ + }}}$
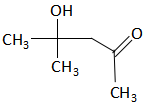 $\xrightarrow{{{I_2}/NaOH}}?$
$\xrightarrow{{{I_2}/NaOH}}?$

Answer
576.3k+ views
Hint: When the alkyne is reacted with $NaN{H_2}$ , it extracts one proton from the ethyne and the carbanion formed acts as nucleophile and attacks for an electrophilic center. The product in presence of mercuric (II) ion is oxidized and then undergoes aldol condensation. The aldol product next is reacted with the iodine in presence of sodium hydroxide which is the haloform test and one of the products is iodoform.
Complete step by step answer:
(i) When ethyne ($CH \equiv CH$ ) reacts with sodium amide ($NaN{H_2}$), the amide extracts one proton from ethyne to form ammonia and the carbanion of the ethyne. The reaction is as follows:
$CH \equiv CH\xrightarrow{{NaN{H_2}}}CH \equiv {C^ - } + N{H_3}$
(ii) The carbanion formed acts as a nucleophile and attacks the electrophilic center of the methyl bromide to produce 1-propyne as the major product. The reaction is as follows:
$CH \equiv {C^ - }\xrightarrow{{C{H_3}Br}}CH \equiv C - C{H_3} + B{r^ - }$
(iii) The 1-propyne formed in the reaction undergoes oxidation reaction in the presence of acidified mercuric (II) ion solution to give acetone in the product. The reaction is as follows:
$CH \equiv C - C{H_3}\xrightarrow{{H{g^{2 + }}/{H^ + }}}$ $C{H_3} - C( = O) - C{H_3}$
(iv) Acetone when reacts with an acidified solution rich in proton, aldol condensation of the reactant takes place. The reaction is as follows:
$2C{H_3} - C( = O) - C{H_3}\xrightarrow{{{H^ + }}}{(C{H_3})_2}C(OH) - C{H_2} - C( = O) - C{H_3}$
(v) The aldol product formed in the above reaction undergoes the iodoform reaction with iodine in the presence of sodium hydroxide, it gives iodoform.
${(C{H_3})_2}C(OH) - C{H_2} - C( = O) - C{H_3}\xrightarrow{{{I_2}/NaOH}}{(C{H_3})_2}C(OH) - C{H_2} - CO{O^ - } + CH{I_3}$
Thus, the overall complete equation is as follows:
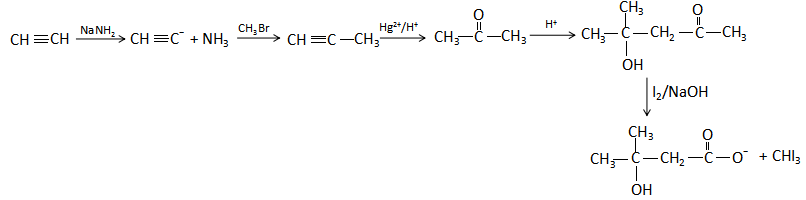
Note: Iodoform test is used to check the presence of carbonyl compounds with the structure \[R - CO - C{H_3}\] or alcohols with the structure \[R - CH\left( {OH} \right) - C{H_3}\] in a given unknown substance. The reaction of iodine, a base and a methyl ketone gives a yellow precipitate along with an antiseptic smell.
Complete step by step answer:
(i) When ethyne ($CH \equiv CH$ ) reacts with sodium amide ($NaN{H_2}$), the amide extracts one proton from ethyne to form ammonia and the carbanion of the ethyne. The reaction is as follows:
$CH \equiv CH\xrightarrow{{NaN{H_2}}}CH \equiv {C^ - } + N{H_3}$
(ii) The carbanion formed acts as a nucleophile and attacks the electrophilic center of the methyl bromide to produce 1-propyne as the major product. The reaction is as follows:
$CH \equiv {C^ - }\xrightarrow{{C{H_3}Br}}CH \equiv C - C{H_3} + B{r^ - }$
(iii) The 1-propyne formed in the reaction undergoes oxidation reaction in the presence of acidified mercuric (II) ion solution to give acetone in the product. The reaction is as follows:
$CH \equiv C - C{H_3}\xrightarrow{{H{g^{2 + }}/{H^ + }}}$ $C{H_3} - C( = O) - C{H_3}$
(iv) Acetone when reacts with an acidified solution rich in proton, aldol condensation of the reactant takes place. The reaction is as follows:
$2C{H_3} - C( = O) - C{H_3}\xrightarrow{{{H^ + }}}{(C{H_3})_2}C(OH) - C{H_2} - C( = O) - C{H_3}$
(v) The aldol product formed in the above reaction undergoes the iodoform reaction with iodine in the presence of sodium hydroxide, it gives iodoform.
${(C{H_3})_2}C(OH) - C{H_2} - C( = O) - C{H_3}\xrightarrow{{{I_2}/NaOH}}{(C{H_3})_2}C(OH) - C{H_2} - CO{O^ - } + CH{I_3}$
Thus, the overall complete equation is as follows:
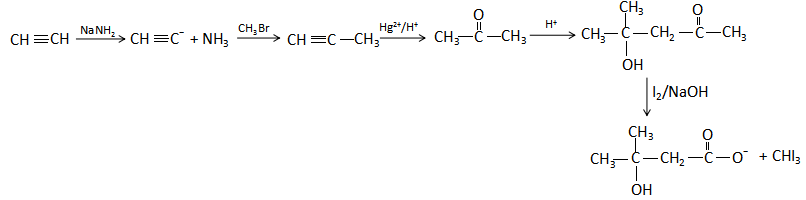
Note: Iodoform test is used to check the presence of carbonyl compounds with the structure \[R - CO - C{H_3}\] or alcohols with the structure \[R - CH\left( {OH} \right) - C{H_3}\] in a given unknown substance. The reaction of iodine, a base and a methyl ketone gives a yellow precipitate along with an antiseptic smell.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




