
Complete the below reaction by providing RHS part,
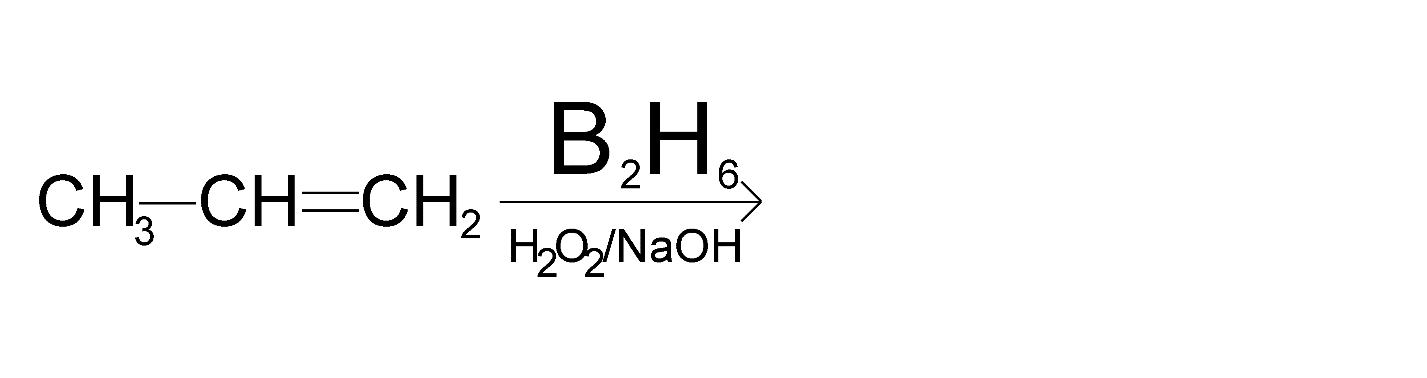
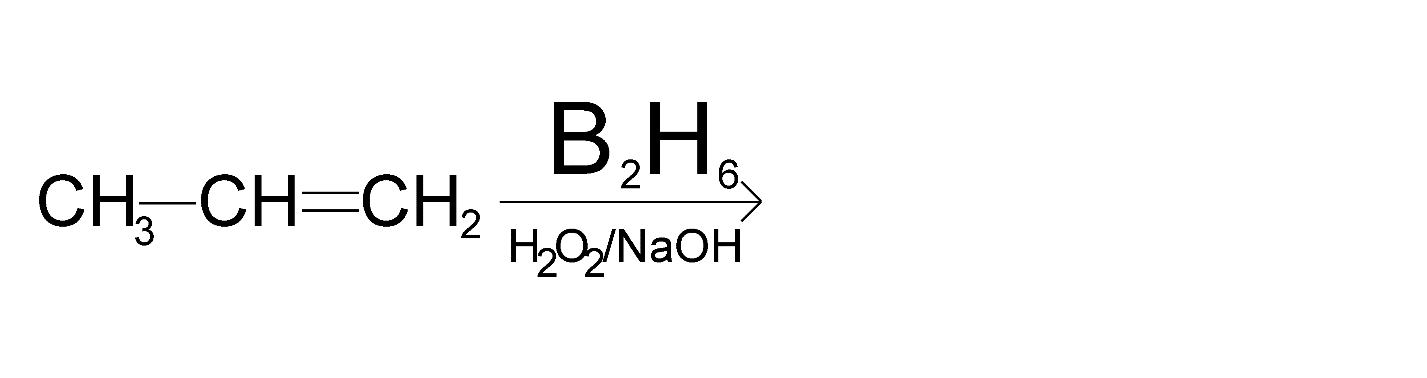
Answer
550.5k+ views
Hint: In hydroboration reaction ${B_2}{H_6}$ in the presence of $NaOH$ and ${H_2}{O_2}$ will convert the given compound, i.e. propene into propanol. In general, hydroboration converts alkene into alcohol.
Complete answer:
Let’s start by discussing the hydroboration oxidation reaction.
Hydroboration-Oxidation is a two-step path used for alcohol production. The reaction proceeds in the Anti-Markovnikov way, where the hydrogen (from $B{H_3}$ or $BH{R_2}$ ) binds to the more substituted carbon and the boron in the alkene double bond binds to the least substituted carbon. Also, by accepting two electrons in its empty p orbital from an electron-rich alkene, the borane acts as a Lewis Anti-Markovnikov acid. This process allows boron to have an electron octet.
A very interesting feature of this process is that no activation by a catalyst is required. The mechanism of anti-Markovnikov hydroboration has both hydrogenation and electrophilic addition elements and is stereospecific (syn addition), which means that the hydroboration takes place on the same side of the double bond, leading to cis stereochemistry.
The hydroboration reaction in propene will be as shown below
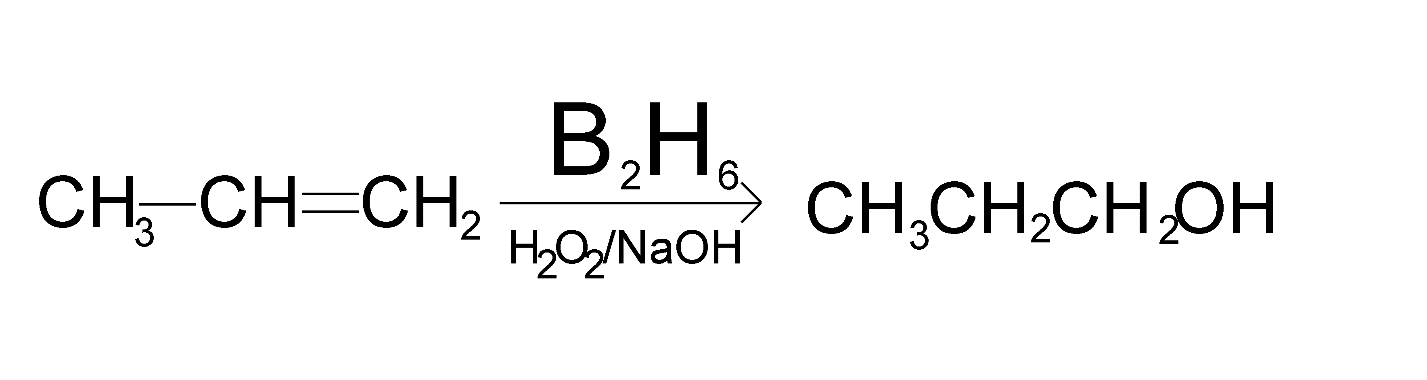
So, the hydroboration reaction of propene will produce propanol.
Note:
A little bit about the structure and properties of the borane molecule is very important to understand. Borane occurs naturally as a very toxic gas, and the general formula ${B_2}{H_6}$ (diborane) exists as a dimer. Additionally, the ${B_2}{H_6}$ dimer spontaneously ignites in the air. Borane is commercially available in ether and tetrahydrofuran ( THF). Borane can exist as a lewis acid-base complex in these solutions, allowing an electron octet to be present in boron.
Complete answer:
Let’s start by discussing the hydroboration oxidation reaction.
Hydroboration-Oxidation is a two-step path used for alcohol production. The reaction proceeds in the Anti-Markovnikov way, where the hydrogen (from $B{H_3}$ or $BH{R_2}$ ) binds to the more substituted carbon and the boron in the alkene double bond binds to the least substituted carbon. Also, by accepting two electrons in its empty p orbital from an electron-rich alkene, the borane acts as a Lewis Anti-Markovnikov acid. This process allows boron to have an electron octet.
A very interesting feature of this process is that no activation by a catalyst is required. The mechanism of anti-Markovnikov hydroboration has both hydrogenation and electrophilic addition elements and is stereospecific (syn addition), which means that the hydroboration takes place on the same side of the double bond, leading to cis stereochemistry.
The hydroboration reaction in propene will be as shown below
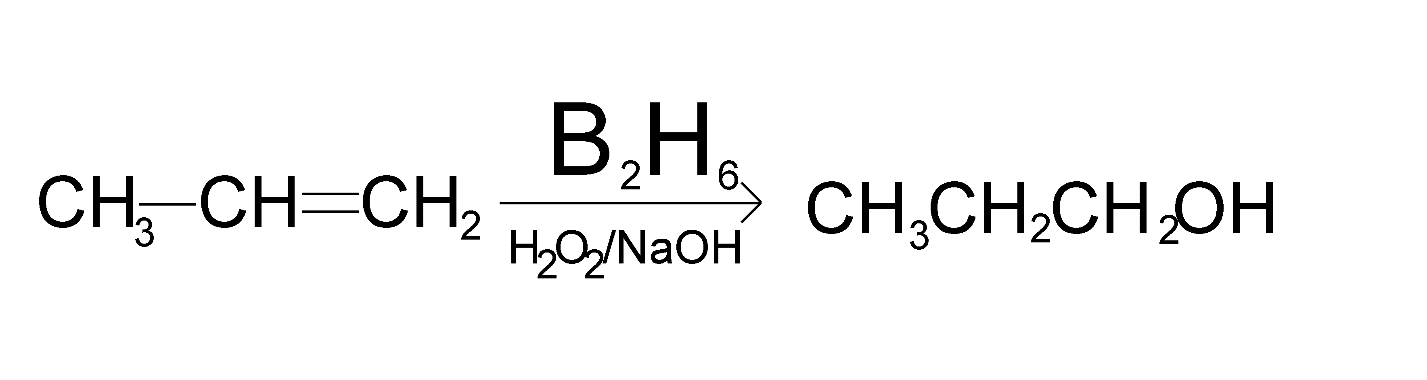
So, the hydroboration reaction of propene will produce propanol.
Note:
A little bit about the structure and properties of the borane molecule is very important to understand. Borane occurs naturally as a very toxic gas, and the general formula ${B_2}{H_6}$ (diborane) exists as a dimer. Additionally, the ${B_2}{H_6}$ dimer spontaneously ignites in the air. Borane is commercially available in ether and tetrahydrofuran ( THF). Borane can exist as a lewis acid-base complex in these solutions, allowing an electron octet to be present in boron.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




