
Compare the basic strengths of the compounds given:
(I) $E{{t}_{3}}N$
(II)
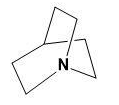
(III)
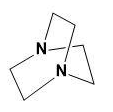
(A) (I) > (II) > (III)
(B) (II) > (I) > (III)
(C) (II) > (III) > (I)
(D) (III) > (II) > (I)


Answer
566.7k+ views
Hint: Basicity of any compound depends upon many factors as the ability to donate electron, show +I effect, not to show -I effect, etc.
Here, depending upon some factors we will discuss and give the order of basicity.
Complete step by step answer:
Let us see the required parameters to solve the given illustration,
Electron withdrawing group-
The presence of EWG shows that the group can withdraw electrons from the system i.e. represents -I effect.
Electron donating group-
The presence of EDG shows that the group can donate electrons to the system i.e. represents +I effect,
Inductive effect plays a very important role in deciding the acidity and basicity of the molecule. We can represent that easily by,
$Basicity\propto +I$ and $Basicity\propto \frac{1}{-I}$ .
Also, basicity, in general, is the ability to donate electrons to the system.
Keeping these points in mind let us move towards the illustration;
We have,
1.$E{{t}_{3}}N$-
Here, Et represents the ethyl group i.e. $C{{H}_{3}}-C{{H}_{2}}-$ group which is the electron donating group showing +I effect. And as from above basicity is directly proportional to the +I effect thus, we can say that $E{{t}_{3}}N$ is highly basic.
2.
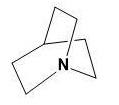
Here, the lone pair present on N can be donated to ${{H}^{+}}$ forming $R-{{N}^{+}}{{H}_{3}}$ which is highly unstable. Thus, this will be less basic.
3.
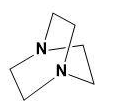
Here, the two N atoms are forming a bridge and both will show -I effect which from the above explanation shows the least basicity.
Therefore, the basicity order will be (I) > (II) > (III).
Hence, option (A) is correct.
Note: Do note that the basicity is dependent on different factors for every different compound but those factors end up to one common thing which is empirical i.e. the ability to donate electrons (basicity).
Here, depending upon some factors we will discuss and give the order of basicity.
Complete step by step answer:
Let us see the required parameters to solve the given illustration,
Electron withdrawing group-
The presence of EWG shows that the group can withdraw electrons from the system i.e. represents -I effect.
Electron donating group-
The presence of EDG shows that the group can donate electrons to the system i.e. represents +I effect,
Inductive effect plays a very important role in deciding the acidity and basicity of the molecule. We can represent that easily by,
$Basicity\propto +I$ and $Basicity\propto \frac{1}{-I}$ .
Also, basicity, in general, is the ability to donate electrons to the system.
Keeping these points in mind let us move towards the illustration;
We have,
1.$E{{t}_{3}}N$-
Here, Et represents the ethyl group i.e. $C{{H}_{3}}-C{{H}_{2}}-$ group which is the electron donating group showing +I effect. And as from above basicity is directly proportional to the +I effect thus, we can say that $E{{t}_{3}}N$ is highly basic.
2.

Here, the lone pair present on N can be donated to ${{H}^{+}}$ forming $R-{{N}^{+}}{{H}_{3}}$ which is highly unstable. Thus, this will be less basic.
3.

Here, the two N atoms are forming a bridge and both will show -I effect which from the above explanation shows the least basicity.
Therefore, the basicity order will be (I) > (II) > (III).
Hence, option (A) is correct.
Note: Do note that the basicity is dependent on different factors for every different compound but those factors end up to one common thing which is empirical i.e. the ability to donate electrons (basicity).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




