
Compare S-S bond length form the following molecules.
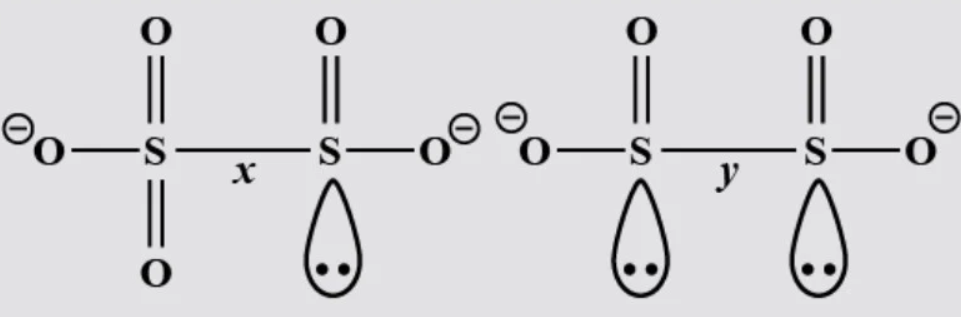
A- $x\quad >\quad y$
B- $y\quad >\quad x$
C- $x\quad =\quad y$
D- None of these

Answer
584.7k+ views
Hint: We do not need to calculate the exact value of bond lengths. We just need to find the relation between their bond lengths. Think about the factors affecting bond length. Such as charge stability and resonance.
Complete step by step answer: We know that both ions contain Sulphur atoms. Exact bond lengths cannot be calculated directly. There are specific methods to calculate given bond length. We can find a relationship between 2 bond lengths.
In the first compound where one Sulphur atom connected to 3 oxygen atoms and another Sulphur atom connected to 2 oxygen atoms. Sulphur atoms connected to 3 oxygen atoms develop some partial positive charge. And this attracts the lone pair of electrons on another Sulphur atom as a result bond order of S-S bond increases partially. We know that as bond order increases bond length decreases. So, the bond length of S-S will be shorter than usual bond length.
In the second compound, both Sulphur atoms are connected to 2 oxygen atoms each. And each Sulphur atom has a lone pair of electrons on them. There will be some electronic repulsions between these 2 Sulphur atoms. As a result, the S-S bond length will increase.
Therefore, $y\quad >\quad x$. Option B is correct.
Note: We may generally think that both bonds are single so both have the same length but other factors influence bond length. Sulphur oxygen bond length is the same for all S-O bonds whereas they are different in the first compound.
Complete step by step answer: We know that both ions contain Sulphur atoms. Exact bond lengths cannot be calculated directly. There are specific methods to calculate given bond length. We can find a relationship between 2 bond lengths.
In the first compound where one Sulphur atom connected to 3 oxygen atoms and another Sulphur atom connected to 2 oxygen atoms. Sulphur atoms connected to 3 oxygen atoms develop some partial positive charge. And this attracts the lone pair of electrons on another Sulphur atom as a result bond order of S-S bond increases partially. We know that as bond order increases bond length decreases. So, the bond length of S-S will be shorter than usual bond length.
In the second compound, both Sulphur atoms are connected to 2 oxygen atoms each. And each Sulphur atom has a lone pair of electrons on them. There will be some electronic repulsions between these 2 Sulphur atoms. As a result, the S-S bond length will increase.
Therefore, $y\quad >\quad x$. Option B is correct.
Note: We may generally think that both bonds are single so both have the same length but other factors influence bond length. Sulphur oxygen bond length is the same for all S-O bonds whereas they are different in the first compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




