
What is the common name of $ 2 - methyl - propanal $ ?
Answer
521.1k+ views
Hint : $ 2 - methyl - propanal $ is an organic compound with molecular formula $ {C_4}{H_8}O $ . It is a $ 3 $ carbon compound (propane) having methyl group on one of the carbon and $ {4^{th}} $ carbon is an aldehyde group $ CHO $ , so it becomes a short-chain aldehydic compound. It is a colorless volatile liquid.
Complete Step By Step Answer:
There are two ways to name an organic compound
1. IUPAC name and
2. common name
This name $ 2 - methyl - propanal $ is an IUPAC name of the compound
Common names-These are widely used today. Common names use some prefixes like: iso-, sec-, tert-, and neo-. And the prefixes are placed in front of the alkane name that indicates the total number of carbons.
Prefix iso- means isomer is used when there is a methyl group on the second carbon of the compound. As in the case of\[2 - methylbutane\]where $ {2^{nd}} $ carbon has a methyl group.
Prefix sec- stands for secondary. It is used when one carbon atom is attached to two other carbon atoms.
tert- stand for tertiary. It is used when one carbon is attached to three other carbon atoms
neo-this is used when the second-last carbon of a compound is trisubstituted.
So now let us look into our given compound and try to give it a common name
So our given compound is $ 2 - methyl - propanal $ and its structure is:
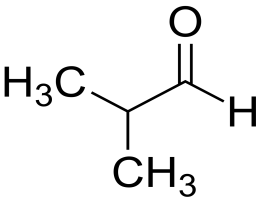
As we can see here the second carbon has a methyl group so it will have a prefix iso- so before the name of the compound, iso- will come. There is an aldehyde group attached at the end, so in the common name -aldehyde is added at the end of the name of the parent chain i.e. it is used as a suffix. Now count the total number of carbon atoms including the methyl and the aldehyde group so by counting this we get butane as the parent chain. And by adding a suffix it will become butyraldehyde.
Hence by considering the prefix, suffix, and parent chain we conclude that the common name of the compound is Isobutyraldehyde.
Therefore the common name of $ 2 - methyl - propanal $ is Isobutyraldehyde.
Note :
IUPAC nomenclature has certain rules. By following those rules one can easily name any organic compound. There are no rules that govern common names by simply adding the prefix and suffix and counting the total carbons of a compound common name can be given.
Complete Step By Step Answer:
There are two ways to name an organic compound
1. IUPAC name and
2. common name
This name $ 2 - methyl - propanal $ is an IUPAC name of the compound
Common names-These are widely used today. Common names use some prefixes like: iso-, sec-, tert-, and neo-. And the prefixes are placed in front of the alkane name that indicates the total number of carbons.
Prefix iso- means isomer is used when there is a methyl group on the second carbon of the compound. As in the case of\[2 - methylbutane\]where $ {2^{nd}} $ carbon has a methyl group.
Prefix sec- stands for secondary. It is used when one carbon atom is attached to two other carbon atoms.
tert- stand for tertiary. It is used when one carbon is attached to three other carbon atoms
neo-this is used when the second-last carbon of a compound is trisubstituted.
So now let us look into our given compound and try to give it a common name
So our given compound is $ 2 - methyl - propanal $ and its structure is:

As we can see here the second carbon has a methyl group so it will have a prefix iso- so before the name of the compound, iso- will come. There is an aldehyde group attached at the end, so in the common name -aldehyde is added at the end of the name of the parent chain i.e. it is used as a suffix. Now count the total number of carbon atoms including the methyl and the aldehyde group so by counting this we get butane as the parent chain. And by adding a suffix it will become butyraldehyde.
Hence by considering the prefix, suffix, and parent chain we conclude that the common name of the compound is Isobutyraldehyde.
Therefore the common name of $ 2 - methyl - propanal $ is Isobutyraldehyde.
Note :
IUPAC nomenclature has certain rules. By following those rules one can easily name any organic compound. There are no rules that govern common names by simply adding the prefix and suffix and counting the total carbons of a compound common name can be given.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




