
Comment on the structure of liquid ${{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}$.
Answer
584.7k+ views
Hint: Iodine and chlorine both belong to the same group i.e. halogen group. ${{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}$is formed by two molecules of ${\text{IC}}{{\text{l}}_3}$. This is a complex compound formed by ${\text{IC}}{{\text{l}}_3}$. The two chlorine atoms of ${{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}$are linking atoms.
Complete step by step answer:
Covalent bond: Those bonds between the atoms in which both the atoms share the electrons to each other.
Coordinate covalent bond: The bond in which the electron is donated by one atom but shared by both the atoms.
${{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}$is formed by two molecules of iodine trichloride. Iodine trichloride is the interhalogen of iodine with chlorine. Here iodine is at centre because its size is large as compared to the size of chlorine. In the structure of ${{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}$two chlorine atoms are linking atoms. Linking atoms are those atoms which are linked to two atoms one by covalent bond and other by the bond in which the electron is donated by one atom but shared by both the atoms i.e. coordinate covalent bond. So they have different angles between the iodine- chlorine atoms. Because all the chlorine atoms present in the ${{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}$molecule is not the same. So they have different bond lengths and angles.
The structure of the ${{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}$molecule is as follows:
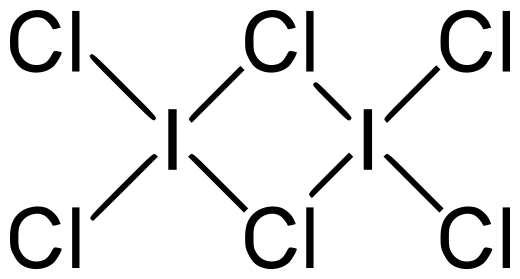
In this structure the chlorine are of two types: the one which is connected to only one iodine atom and the second one is that which is connected to two iodine atoms.
Note:
The structure can be studied by using molecular orbital theory (M.O.T). It can be defined as how electrons or atoms are assigned in the molecule. Although electrons are not moving there but they are treated as moving under the influence of atomic nuclei in the whole molecule.
Complete step by step answer:
Covalent bond: Those bonds between the atoms in which both the atoms share the electrons to each other.
Coordinate covalent bond: The bond in which the electron is donated by one atom but shared by both the atoms.
${{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}$is formed by two molecules of iodine trichloride. Iodine trichloride is the interhalogen of iodine with chlorine. Here iodine is at centre because its size is large as compared to the size of chlorine. In the structure of ${{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}$two chlorine atoms are linking atoms. Linking atoms are those atoms which are linked to two atoms one by covalent bond and other by the bond in which the electron is donated by one atom but shared by both the atoms i.e. coordinate covalent bond. So they have different angles between the iodine- chlorine atoms. Because all the chlorine atoms present in the ${{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}$molecule is not the same. So they have different bond lengths and angles.
The structure of the ${{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}$molecule is as follows:

In this structure the chlorine are of two types: the one which is connected to only one iodine atom and the second one is that which is connected to two iodine atoms.
Note:
The structure can be studied by using molecular orbital theory (M.O.T). It can be defined as how electrons or atoms are assigned in the molecule. Although electrons are not moving there but they are treated as moving under the influence of atomic nuclei in the whole molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




