
$C{{O}_{2}}$ is not iso-structural with $S{{O}_{2}}$ . Explain.
Answer
576.6k+ views
HINT: To find the answer to this question start by finding out the coordination number of them. They will be iso-structural if they have the same structure i.e. similar bond angles and shape. Due to the presence of lone-pair one of them will have a slightly different shape.
Complete step by step solution:
We call two compounds iso-structural when they have the same chemical structure but not necessarily the same chemical elements involved. To find the structures of compounds, we generally opt for the VSEPR theory i.e. the valence shell electron pair repulsion theory.
Let us discuss the structure of carbon dioxide first on the basis of VSEPR. In carbon dioxide, we have one atom of carbon bonded to one molecule of oxygen. We know that the atomic number of carbon is 4. Thus, we know that it has 4 valence electrons in its outermost shell.
The two oxygen atoms donate an electron pair each i.e. 2 electrons thus, for 2 oxygen atoms i.e. a molecule of oxygen we have a total contribution of 4 electrons. Therefore, the total number of electrons that we have here is 8. So the number of electrons pairs is 4. But carbon dioxide has two double bonds i.e. the two oxygen atoms are bonded to the central carbon atom through a double bond.
-Therefore, the total number of electron pairs comes out to be 2 i.e. its coordination number is 2 which means it has a linear shape.
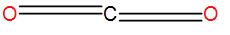
-The bond angle is ${{180}^{{}^\circ }}$ here and the hybridization of the central atom is sp.
-Now, let us try to find the shape of sulphur dioxide according to the VSEPR theory.
-We know that the atomic number of sulphur is 16 i.e. its electronic configuration is 2, 8, 6. It has 6 electrons in its valence shell.
-The two oxygen atoms donate an electron pair each i.e. 2 electrons thus, for 2 oxygen atoms i.e. a molecule of oxygen we have a total contribution of 4 electrons.
-Therefore, the total number of electrons is 10 or the number of electron pairs is 5. Now here also the two oxygen atoms are bonded to the central sulphur atom through a double bond.
-Therefore, the total number of electron pairs becomes 3 i.e. the coordination number is 3.
According to the VSEPR theory, for coordination number 3, the geometry is trigonal planar. But here we have a lone pair present and due to lone-pair – bond-pair repulsion between the lone pairs and the bonding pairs of oxygen atoms, the shape becomes bent and the bond angle decrease slightly from the theoretical ${{120}^{{}^\circ }}$ bond angle.
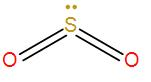
The hybridisation of the central metal is $s{{p}^{2}}$. As we can see from the above discussion that carbon dioxide has a linear shape and a sp hybridisation whereas sulphur dioxide has a bent shape and a $s{{p}^{2}}$ hybridisation therefore, they are not iso-structural.
Note: Although VSEPR is quite successful in determining the geometries of molecules but there are certain drawbacks to this theory. This theory fails to determine the shape of isoelectronic species. It also does not take the relative size of the constituents under consideration. It is unable to explain the atomic orbital overlaps.
Complete step by step solution:
We call two compounds iso-structural when they have the same chemical structure but not necessarily the same chemical elements involved. To find the structures of compounds, we generally opt for the VSEPR theory i.e. the valence shell electron pair repulsion theory.
Let us discuss the structure of carbon dioxide first on the basis of VSEPR. In carbon dioxide, we have one atom of carbon bonded to one molecule of oxygen. We know that the atomic number of carbon is 4. Thus, we know that it has 4 valence electrons in its outermost shell.
The two oxygen atoms donate an electron pair each i.e. 2 electrons thus, for 2 oxygen atoms i.e. a molecule of oxygen we have a total contribution of 4 electrons. Therefore, the total number of electrons that we have here is 8. So the number of electrons pairs is 4. But carbon dioxide has two double bonds i.e. the two oxygen atoms are bonded to the central carbon atom through a double bond.
-Therefore, the total number of electron pairs comes out to be 2 i.e. its coordination number is 2 which means it has a linear shape.
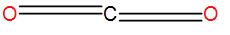
-The bond angle is ${{180}^{{}^\circ }}$ here and the hybridization of the central atom is sp.
-Now, let us try to find the shape of sulphur dioxide according to the VSEPR theory.
-We know that the atomic number of sulphur is 16 i.e. its electronic configuration is 2, 8, 6. It has 6 electrons in its valence shell.
-The two oxygen atoms donate an electron pair each i.e. 2 electrons thus, for 2 oxygen atoms i.e. a molecule of oxygen we have a total contribution of 4 electrons.
-Therefore, the total number of electrons is 10 or the number of electron pairs is 5. Now here also the two oxygen atoms are bonded to the central sulphur atom through a double bond.
-Therefore, the total number of electron pairs becomes 3 i.e. the coordination number is 3.
According to the VSEPR theory, for coordination number 3, the geometry is trigonal planar. But here we have a lone pair present and due to lone-pair – bond-pair repulsion between the lone pairs and the bonding pairs of oxygen atoms, the shape becomes bent and the bond angle decrease slightly from the theoretical ${{120}^{{}^\circ }}$ bond angle.

The hybridisation of the central metal is $s{{p}^{2}}$. As we can see from the above discussion that carbon dioxide has a linear shape and a sp hybridisation whereas sulphur dioxide has a bent shape and a $s{{p}^{2}}$ hybridisation therefore, they are not iso-structural.
Note: Although VSEPR is quite successful in determining the geometries of molecules but there are certain drawbacks to this theory. This theory fails to determine the shape of isoelectronic species. It also does not take the relative size of the constituents under consideration. It is unable to explain the atomic orbital overlaps.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




