
$C{O_2}$ has the same geometry as:
i.$HgC{l_2}$.
ii.$N{O_2}$
iii.$SnC{l_2}$
iv.\[{C_2}{H_2}\]
A.i and ii
B.ii and iv
C.i and iv
D.iii and iv
Answer
585.6k+ views
Hint:$C{O_2}$ has linear geometry as it has two electron groups and no lone pairs. So, we will draw the geometry of $C{O_2}$ and come to know that as it has no lone pair, shape and geometry will be the same that is linear and now we have to see whether other molecules have lone pairs present or not.
Complete step by step answer:
There are two main factors that determine the geometry of a molecule:
The number of bonding electron pairs around the central atom.
These outer shell or valence shell electrons will try to sit themselves as far as possible from one another as to reduce the electron repulsion or as to attain maximum stability.
As to find the molecular geometry firstly we need to draw Lewis dot structure of the molecule.
$C{O_2}$ has linear geometry , because there are no lone pairs that will affect the orientation of the molecule.
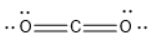
$HgC{l_2}$ has linear geometry , because there are no lone pairs that will affect the orientation of the molecule.
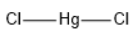
$N{O_2}$ has bent geometry, because there are lone pairs that will affect the orientation of the molecule.
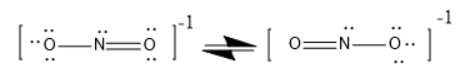
It is in resonance.
$SnC{l_2}$ has linear geometry , because there are lone pair that will affect the orientation of the molecule.
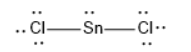
\[{C_2}{H_2}\] has linear geometry , because there are no lone pairs that will affect the orientation of the molecule.
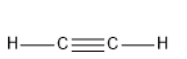
Therefore, option C is correct.
$C{O_2}$ has the same geometry as $HgC{l_2}$and \[{C_2}{H_2}\].
Note:
Geometry of a molecule can be explained by the arrangement of lone pair and bond pairs while shape can be explained by the arrangement of atoms around the atoms, it is not linked with lone pair and hence only deals with bond pairs.
Complete step by step answer:
There are two main factors that determine the geometry of a molecule:
The number of bonding electron pairs around the central atom.
These outer shell or valence shell electrons will try to sit themselves as far as possible from one another as to reduce the electron repulsion or as to attain maximum stability.
As to find the molecular geometry firstly we need to draw Lewis dot structure of the molecule.
$C{O_2}$ has linear geometry , because there are no lone pairs that will affect the orientation of the molecule.
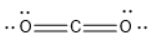
$HgC{l_2}$ has linear geometry , because there are no lone pairs that will affect the orientation of the molecule.
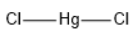
$N{O_2}$ has bent geometry, because there are lone pairs that will affect the orientation of the molecule.
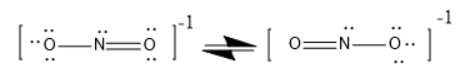
It is in resonance.
$SnC{l_2}$ has linear geometry , because there are lone pair that will affect the orientation of the molecule.
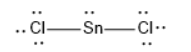
\[{C_2}{H_2}\] has linear geometry , because there are no lone pairs that will affect the orientation of the molecule.
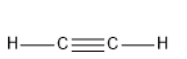
Therefore, option C is correct.
$C{O_2}$ has the same geometry as $HgC{l_2}$and \[{C_2}{H_2}\].
Note:
Geometry of a molecule can be explained by the arrangement of lone pair and bond pairs while shape can be explained by the arrangement of atoms around the atoms, it is not linked with lone pair and hence only deals with bond pairs.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




