
Classify the following as primary, secondary and tertiary alcohols.

Answer
566.7k+ views
Hint: The answer to this question is dependent on the concept of general chemistry that includes the basic definition of primary, secondary and tertiary carbon atoms. It is defined on the basis of the number of carbon atoms attached to that particular carbon.
Complete step by step answer:
We have come across the general concept of chemistry which basically deals with the concept of classification of alkanes into the types of carbon atoms.
Now, let us refresh those definitions which will help us to lead to the correct answer.
- By definition of primary carbons, it is nothing but the carbon is said to be primary carbon if that carbon atom is attached to one other carbon atom.
- Secondary carbons are those which are attached to two other carbon atoms
- Tertiary carbon atoms are those in which the carbon atom is attached to three other carbon atoms.
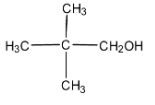
Now, in the above molecule given, the three methyl groups that are $-C{{H}_{3}}$ groups attached to one carbon each are the primary carbon atoms as all three are singly attached to one carbon atom.
In the carbon atom attached with one alcohol group that is $-C{{H}_{2}}OH$, the carbon here is attached to one carbon atom. Thus, this becomes the primary alcohol group.
Therefore, the correct answer is only one primary alcohol group is present in the given compound.
Note: There is also a quaternary carbon atom that is the carbon attached to four other carbon atoms and is in the form of single carbon with no hydrogen attached. Here, in the above question, the carbon attached to three methyl groups and one methanol group is the quaternary carbon.
Complete step by step answer:
We have come across the general concept of chemistry which basically deals with the concept of classification of alkanes into the types of carbon atoms.
Now, let us refresh those definitions which will help us to lead to the correct answer.
- By definition of primary carbons, it is nothing but the carbon is said to be primary carbon if that carbon atom is attached to one other carbon atom.
- Secondary carbons are those which are attached to two other carbon atoms
- Tertiary carbon atoms are those in which the carbon atom is attached to three other carbon atoms.
Now, in the above molecule given, the three methyl groups that are $-C{{H}_{3}}$ groups attached to one carbon each are the primary carbon atoms as all three are singly attached to one carbon atom.
In the carbon atom attached with one alcohol group that is $-C{{H}_{2}}OH$, the carbon here is attached to one carbon atom. Thus, this becomes the primary alcohol group.
Therefore, the correct answer is only one primary alcohol group is present in the given compound.
Note: There is also a quaternary carbon atom that is the carbon attached to four other carbon atoms and is in the form of single carbon with no hydrogen attached. Here, in the above question, the carbon attached to three methyl groups and one methanol group is the quaternary carbon.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




