
\[C{l_2}{O_7}\] can be regarded as an anhydride of
A.Hypochlorous acid
B.Chlorous acid
C.Chloric acid
D.Perchloric acid
Answer
583.2k+ views
Hint: An oxo acid is an acid that contains Oxygen and at least one other element. It is also known as Oxyacid. The Oxoacids of Halogen include Hydrohalic acid, Halous acid, Halic acid and Phthalic acid.
Complete step by step answer:
Except for fluorine, the halogens have an extensive oxoacid. The Oxoacids of Halogen include Hydrohalic acid, Halous acid, Halic acid and Phthalic acid. Different oxoacids of Chlorine include Hypochlorous acid, Chlorous acid, Chloric acid, Perchloric acid.
Hypochlorous acid (HOCl) and Chlorous acid \[\left( {HCl{O_2}} \right)\] are weak monobasic acid. Chloric acid is a strong monobasic acid.
Among these, Perchloric acid is a very strong Monobasic acid. It is colourless, in the anhydrous form. Perchloric acid is unstable and decomposes on standing
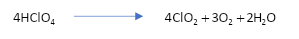
In HClO4 Chlorine is in a state of sp3 hybridization and it has a tetrahedral structure.

\[C{l_2}{O_7}\] on reaction with water forming \[HCl{O_4}\] . Thus, we can say that \[HCl{O_4}\] is an anhydride of Perchloric acid.
Hence, the Correct answer is option (D) i.e Perchloric acid.
Note: Acidic strength of oxoacids of Chlorine increases with increase in the oxidation number of Chlorine.
\[HCl{O_4} < HCl{O_2} < HCl{O_3} < HCl{O_4}\]
Chlorine is present in +7 oxidation state in \[HCl{O_4}\] . This can be explained by the relative strength of conjugate bases. As the stability of the Conjugate base increases lower will be the basic strength and stronger will be the acid. Stability of conjugate base follows the order
\[Cl{O^ - } < ClO{2^ - } < ClO{3^ - } < ClO{4^ - }\]
This is because as the number of Oxygen atoms increases the dispersal of negative charge through \[d\pi - p\pi \] back bonding will be high.
Basic strength of conjugate base decreases in the order \[Cl{O^ - } > Cl{O_2}^ - > Cl{O_3}^ - > Cl{O_4}^ - \] .
Complete step by step answer:
Except for fluorine, the halogens have an extensive oxoacid. The Oxoacids of Halogen include Hydrohalic acid, Halous acid, Halic acid and Phthalic acid. Different oxoacids of Chlorine include Hypochlorous acid, Chlorous acid, Chloric acid, Perchloric acid.
Hypochlorous acid (HOCl) and Chlorous acid \[\left( {HCl{O_2}} \right)\] are weak monobasic acid. Chloric acid is a strong monobasic acid.
Among these, Perchloric acid is a very strong Monobasic acid. It is colourless, in the anhydrous form. Perchloric acid is unstable and decomposes on standing
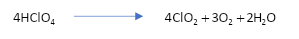
In HClO4 Chlorine is in a state of sp3 hybridization and it has a tetrahedral structure.

\[C{l_2}{O_7}\] on reaction with water forming \[HCl{O_4}\] . Thus, we can say that \[HCl{O_4}\] is an anhydride of Perchloric acid.
Hence, the Correct answer is option (D) i.e Perchloric acid.
Note: Acidic strength of oxoacids of Chlorine increases with increase in the oxidation number of Chlorine.
\[HCl{O_4} < HCl{O_2} < HCl{O_3} < HCl{O_4}\]
Chlorine is present in +7 oxidation state in \[HCl{O_4}\] . This can be explained by the relative strength of conjugate bases. As the stability of the Conjugate base increases lower will be the basic strength and stronger will be the acid. Stability of conjugate base follows the order
\[Cl{O^ - } < ClO{2^ - } < ClO{3^ - } < ClO{4^ - }\]
This is because as the number of Oxygen atoms increases the dispersal of negative charge through \[d\pi - p\pi \] back bonding will be high.
Basic strength of conjugate base decreases in the order \[Cl{O^ - } > Cl{O_2}^ - > Cl{O_3}^ - > Cl{O_4}^ - \] .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




