
$Cis - 2 - butene$ on reaction with bromine in carbon tetrachloride produces mainly.
A) $1 - bromo - 2 - butene$
B) \[{\text{2,3 - dibromobutane}}\]
C) ${\text{meso - 2,3 - dibromobutane}}$
D) $+-2,3 - dibromobutane$
Answer
584.1k+ views
Hint:We know that,
The addition of bromine to the $Cis - 2 - butene$ is clearly determined by the anti-addition and the almost completely restricted rotation of the carbon-carbon double bond of the halonium ion. Therefore, the bromination of $Cis - 2 - butene$ on gives a racemic mixture of (2R, 3R) - and (2S, 3S)-dibromobutane, whereas the bromination of $trans - 2 - butene$ on gives the meso compound.
Complete step by step answer:
We know that the cis and trans isomers are geometrical isomers. Let us discuss the geometric isomer.
Geometric isomers:
Geometric isomers are isomers which arise due to different arrangements of atoms or groups around a bond which has restricted rotation. Geometric isomers are also known as Cis- trans isomerism.
-If the two atoms are locked on the same side of the molecule then it is called cis isomers.
-If the two atoms are locked on the opposite side of the molecule then it is called trans-isomers.
First we see the structure of $Cis - 2 - butene$
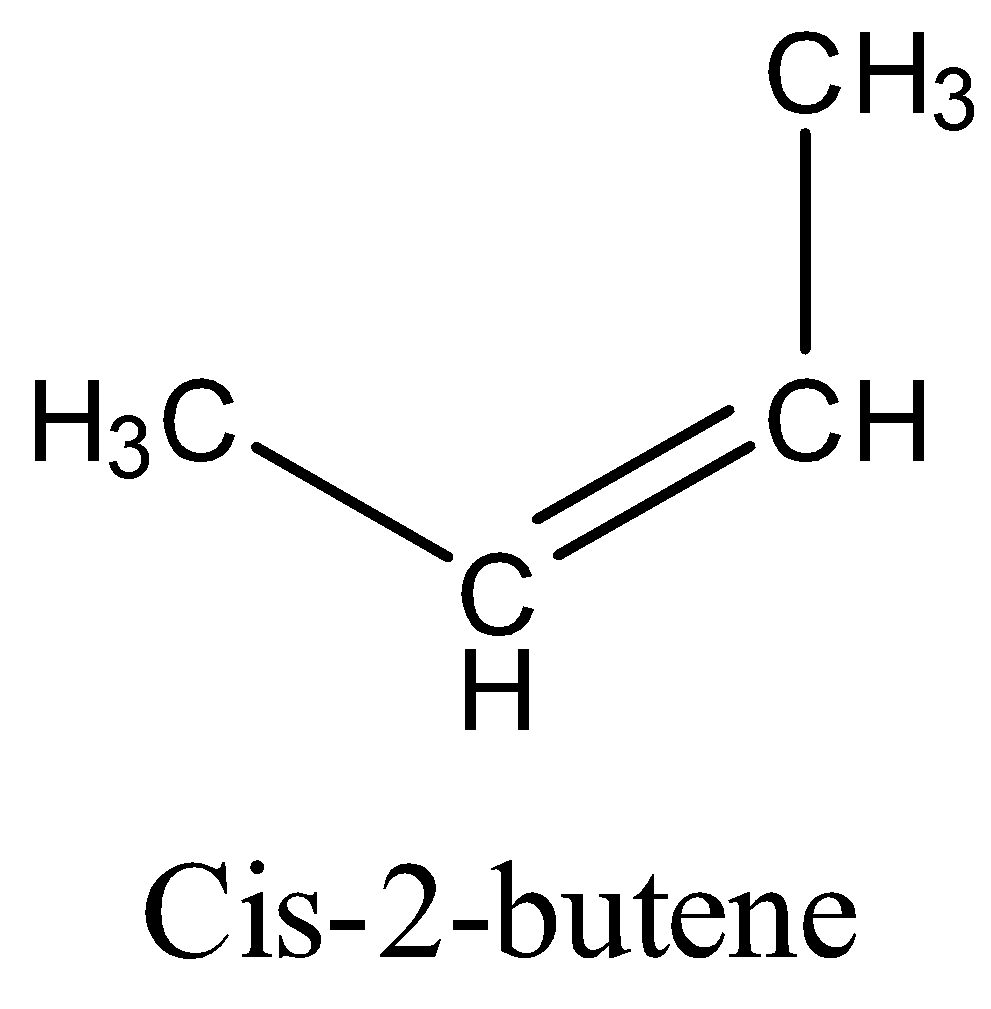
The addition of bromine to $Cis - butene$ on produces an achiral bromonium ion intermediate. Consequent attack of the bromide ion gives the same product because the bromonium ion is symmetrically substituted. The product of the reaction is the racemic mixture of the enantiomeric product $\left( {{\text{2R,3R}}} \right){\text{ - 2,3 - dibromobutane}}\;{\text{and }}\left( {{\text{2S,3S}}} \right){\text{ - 2,3 - dibromobutane}}$.
The reaction is can be written as,
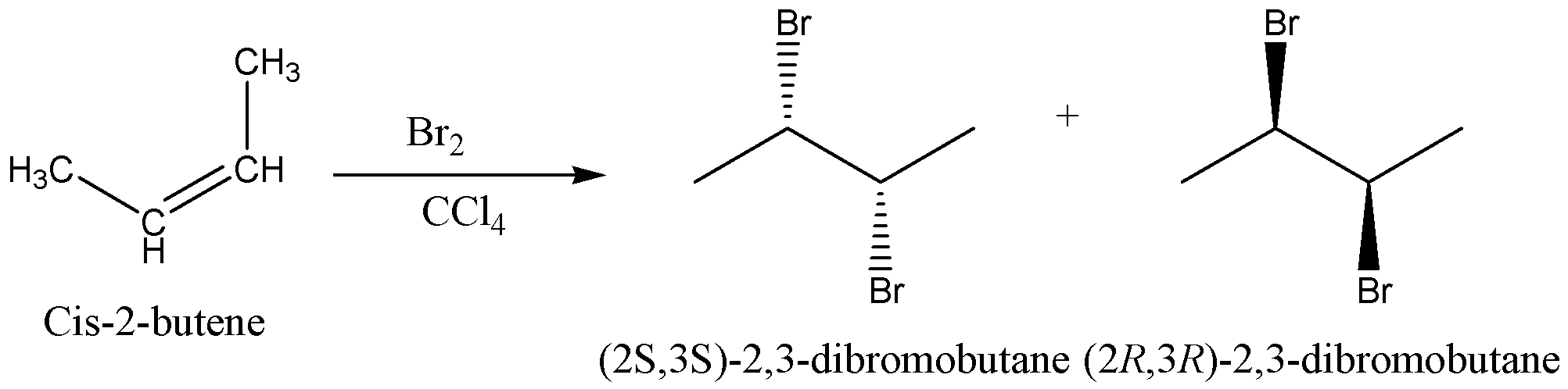
Therefore, the option D is correct.
Note:
On the other hand, a chiral intermediate bromonium ion is formed by the addition of bromine to the trans isomer. The products are meso compounds $\left( {{\text{2S,3R}}} \right){\text{ - 2,3 - dibromobutane}}\;{\text{and }}\left( {{\text{2R,3S}}} \right){\text{ - 2,3 - dibromobutane}}$ and therefore they are identical.
The addition of bromine to the $Cis - 2 - butene$ is clearly determined by the anti-addition and the almost completely restricted rotation of the carbon-carbon double bond of the halonium ion. Therefore, the bromination of $Cis - 2 - butene$ on gives a racemic mixture of (2R, 3R) - and (2S, 3S)-dibromobutane, whereas the bromination of $trans - 2 - butene$ on gives the meso compound.
Complete step by step answer:
We know that the cis and trans isomers are geometrical isomers. Let us discuss the geometric isomer.
Geometric isomers:
Geometric isomers are isomers which arise due to different arrangements of atoms or groups around a bond which has restricted rotation. Geometric isomers are also known as Cis- trans isomerism.
-If the two atoms are locked on the same side of the molecule then it is called cis isomers.
-If the two atoms are locked on the opposite side of the molecule then it is called trans-isomers.
First we see the structure of $Cis - 2 - butene$

The addition of bromine to $Cis - butene$ on produces an achiral bromonium ion intermediate. Consequent attack of the bromide ion gives the same product because the bromonium ion is symmetrically substituted. The product of the reaction is the racemic mixture of the enantiomeric product $\left( {{\text{2R,3R}}} \right){\text{ - 2,3 - dibromobutane}}\;{\text{and }}\left( {{\text{2S,3S}}} \right){\text{ - 2,3 - dibromobutane}}$.
The reaction is can be written as,

Therefore, the option D is correct.
Note:
On the other hand, a chiral intermediate bromonium ion is formed by the addition of bromine to the trans isomer. The products are meso compounds $\left( {{\text{2S,3R}}} \right){\text{ - 2,3 - dibromobutane}}\;{\text{and }}\left( {{\text{2R,3S}}} \right){\text{ - 2,3 - dibromobutane}}$ and therefore they are identical.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




