
Choose the correct option(s) that give(s) an aromatic compound as the major product:
A.
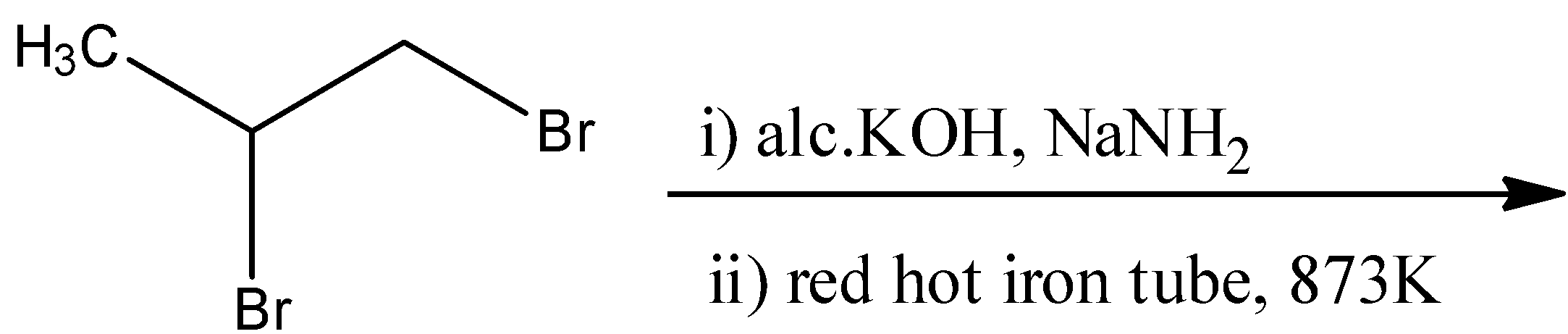
B.
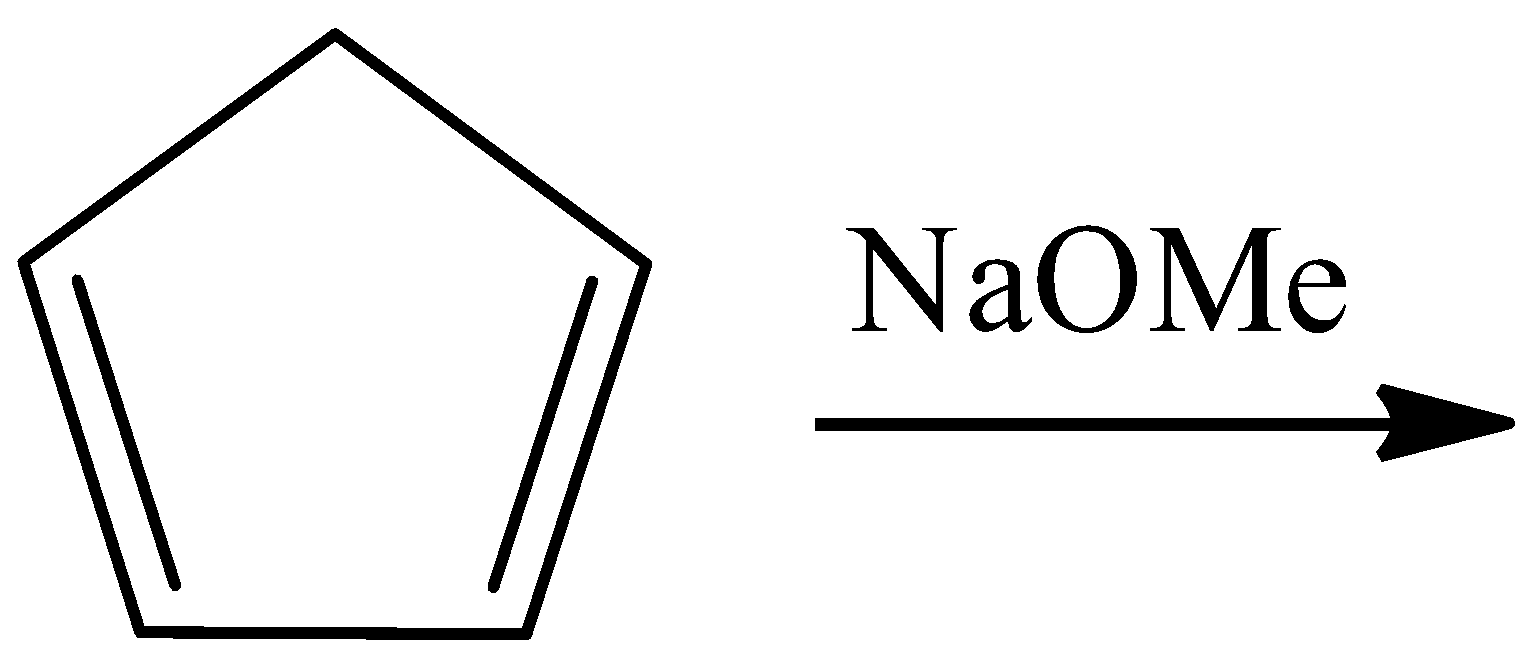
C.
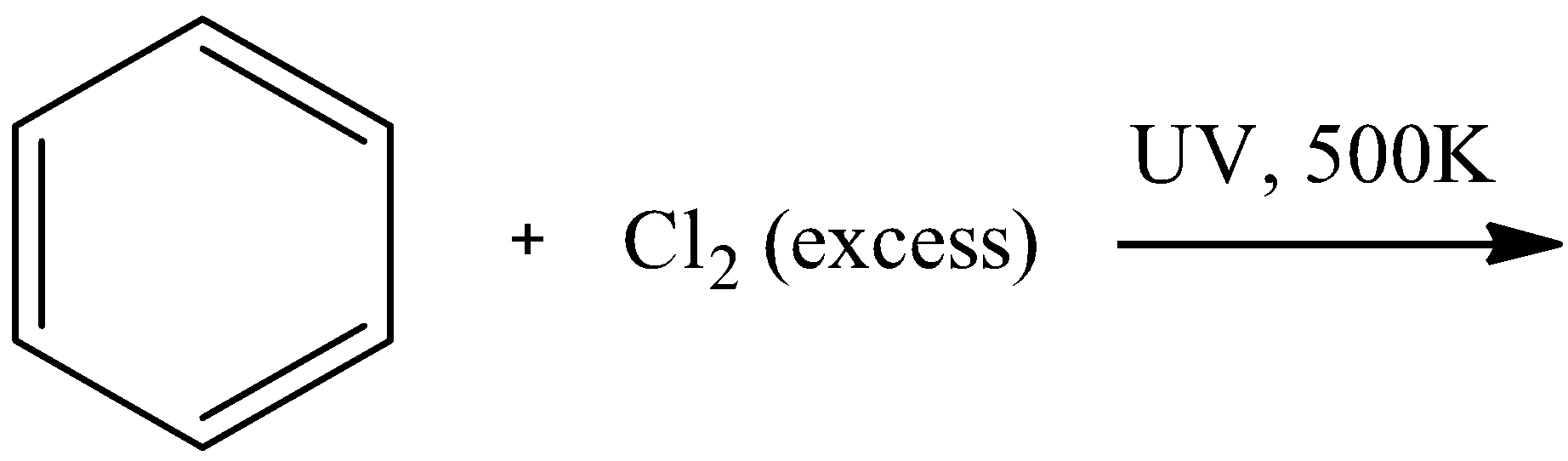
This question has multiple correct options
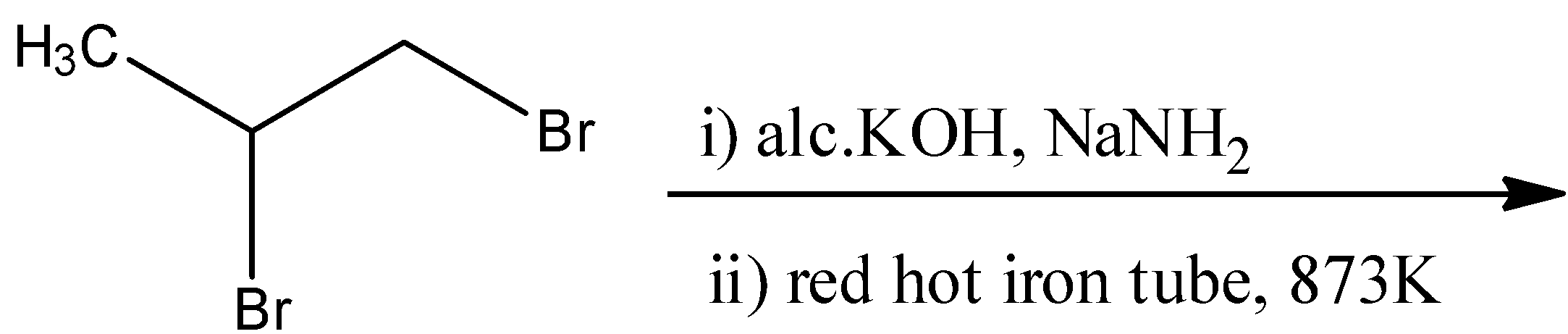

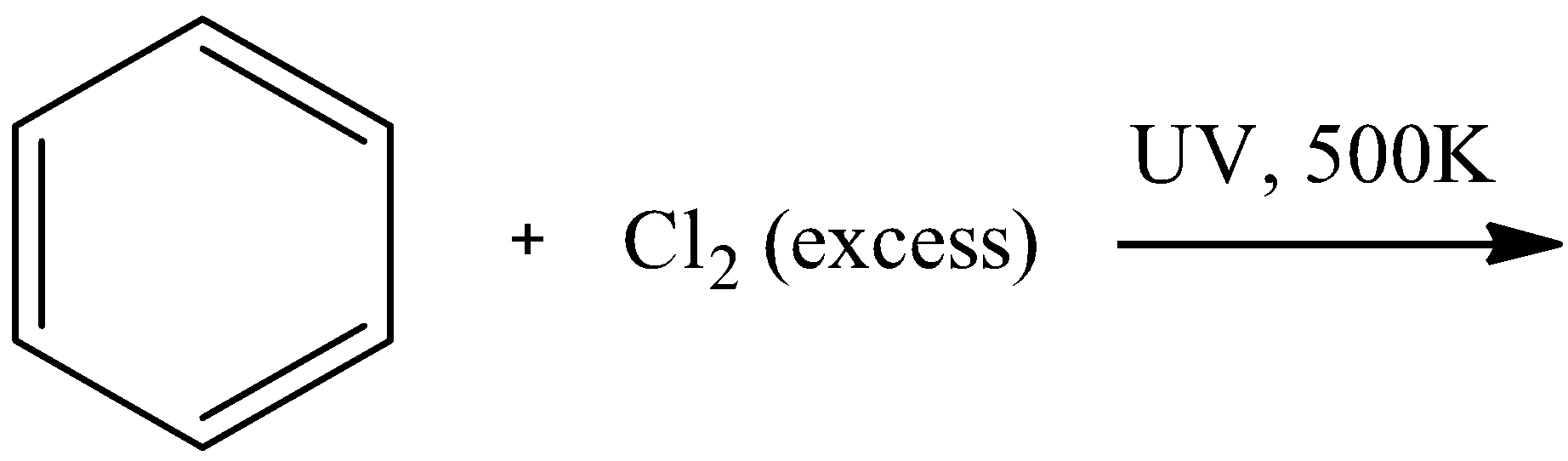
Answer
558.6k+ views
Hint: Predict the products of the reactions given in the options and check if the major product adheres to the rule that defines all aromatic compounds. This rule pertains to the number of pi-bonding electrons that are present in the molecule.
Complete step by step answer:
We know that general rule that is used to determine whether a cyclic molecule is considered to be aromatic or not. Of all the electrons, the number of pi-electrons should be equal to $4n+2$, where $n$ is any natural number. So, the number of pi-electrons can be 6, 10, 14, etc. Now we will look at each of the reactions one by one and check if the products are aromatic or not.
- For option A
The geminal dihalide that is given in the reaction will undergo dehydrohalogenation two times to form an alkyne. The reactant will react with alcoholic $KOH$ in the presence of sodium amide in liquid ammonia to form a propyne molecule. This propyne molecule will then undergo cyclization in a red-hot iron tube to form hexamethylbenzene. The reaction for this is as follows:
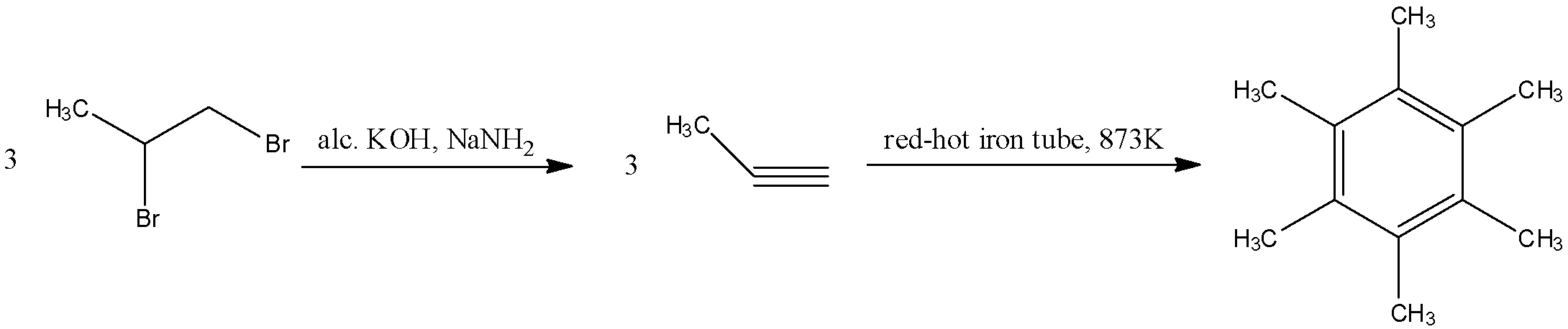
Here, the number of pi-electrons are 6, which fits into our formula of $4n+2$. Hence, this is an aromatic compound.
- For option B
In this reaction, pentene reacts with sodium methoxide to give an ionic species that has a negative charge on the only $s{{p}^{3}}$ hybridized carbon atom in the reactant. This makes that atom partially $s{{p}^{2}}$ hybridized and planar. The reaction is as follows:
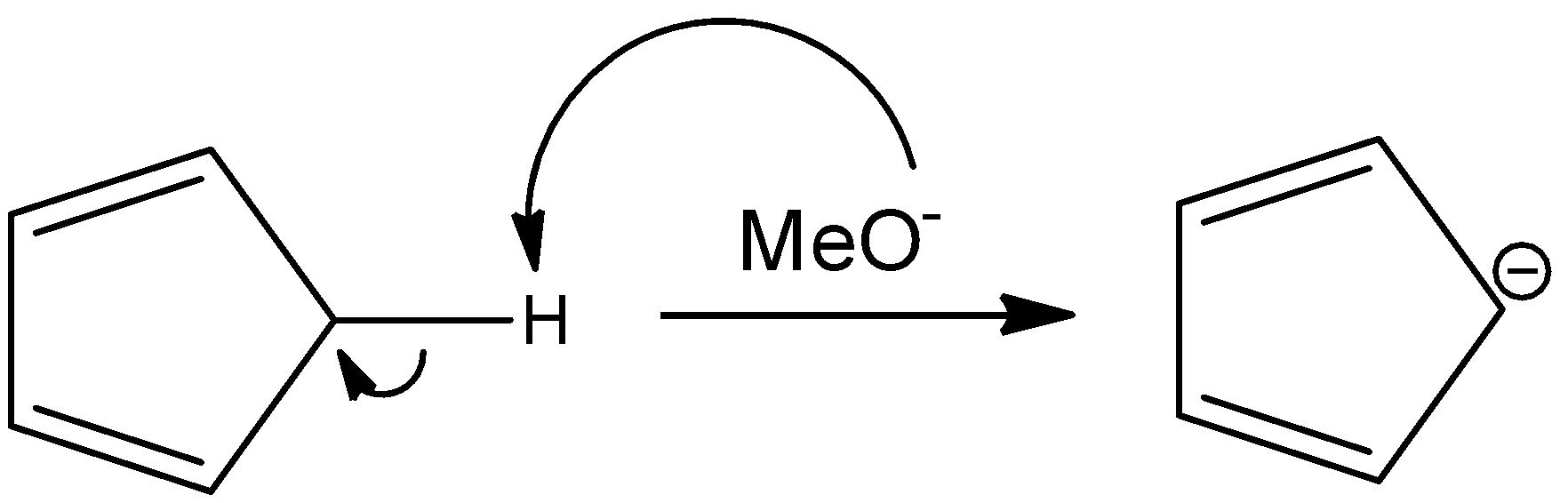
Here again, the number of pi-electrons are 6, the negative charge that is present on the ring will form a pi-bond with any components within the ring and will contribute to the resonating structures of the ring as pi-electrons. Hence, this is also an aromatic compound.
- For option C
We know that the bond between the $Cl$ atoms breaks in ultraviolet light and the formation of free radicals takes place. These free radicals can easily break the double bonds in the benzene ring and cause its halogenation on all the 6 sites possible to form hexachlorocyclohexane. The reaction is as follows:
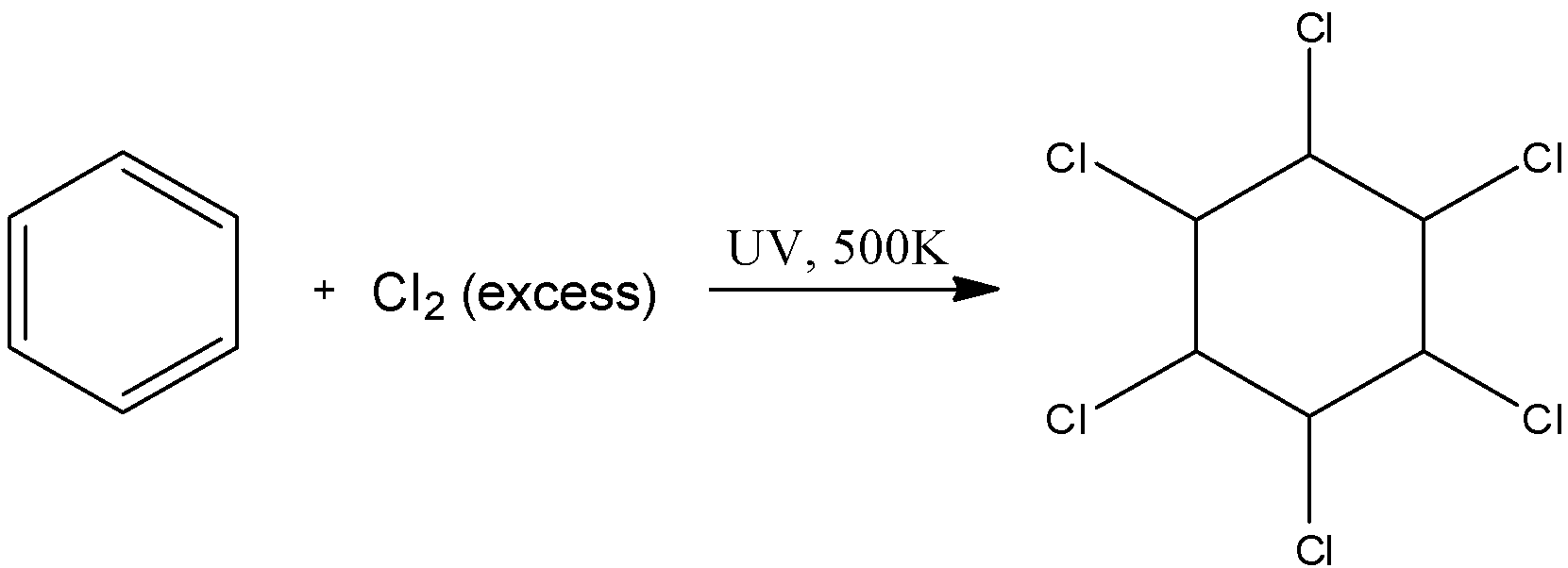
Here, we can see that there are no pi-electrons present. Thus, this is not an aromatic compound.
From the above set of reactions, we can identify that the reactions giving aromatic compounds as major products are options (B) and (C). So the correct answer is “B and C”:
Note: Remember that for a cyclic compound to be aromatic, the presence of double and single bonds in an alternating manner is also necessary. If the molecule satisfies the rule for the pi-electrons then check if the bonds are alternating in nature, if they are not, then the compound is not aromatic.
Complete step by step answer:
We know that general rule that is used to determine whether a cyclic molecule is considered to be aromatic or not. Of all the electrons, the number of pi-electrons should be equal to $4n+2$, where $n$ is any natural number. So, the number of pi-electrons can be 6, 10, 14, etc. Now we will look at each of the reactions one by one and check if the products are aromatic or not.
- For option A
The geminal dihalide that is given in the reaction will undergo dehydrohalogenation two times to form an alkyne. The reactant will react with alcoholic $KOH$ in the presence of sodium amide in liquid ammonia to form a propyne molecule. This propyne molecule will then undergo cyclization in a red-hot iron tube to form hexamethylbenzene. The reaction for this is as follows:

Here, the number of pi-electrons are 6, which fits into our formula of $4n+2$. Hence, this is an aromatic compound.
- For option B
In this reaction, pentene reacts with sodium methoxide to give an ionic species that has a negative charge on the only $s{{p}^{3}}$ hybridized carbon atom in the reactant. This makes that atom partially $s{{p}^{2}}$ hybridized and planar. The reaction is as follows:

Here again, the number of pi-electrons are 6, the negative charge that is present on the ring will form a pi-bond with any components within the ring and will contribute to the resonating structures of the ring as pi-electrons. Hence, this is also an aromatic compound.
- For option C
We know that the bond between the $Cl$ atoms breaks in ultraviolet light and the formation of free radicals takes place. These free radicals can easily break the double bonds in the benzene ring and cause its halogenation on all the 6 sites possible to form hexachlorocyclohexane. The reaction is as follows:

Here, we can see that there are no pi-electrons present. Thus, this is not an aromatic compound.
From the above set of reactions, we can identify that the reactions giving aromatic compounds as major products are options (B) and (C). So the correct answer is “B and C”:
Note: Remember that for a cyclic compound to be aromatic, the presence of double and single bonds in an alternating manner is also necessary. If the molecule satisfies the rule for the pi-electrons then check if the bonds are alternating in nature, if they are not, then the compound is not aromatic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




