
Chlorobenzene is prepared commercially by
Answer
572.1k+ views
Hint: Draw the expanded structure of chlorobenzene. Now try to understand the type of reactions mentioned in the options. With this you can form a clear understanding of the products formed after the respective reactions. Based on this you can determine the process that given chlorobenzene as the major product and thus answer the question.
Complete answer:
The expanded structure for chlorobenzene is :
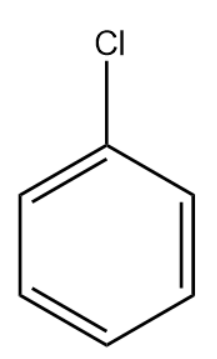
We will now evaluate the processes mentioned in the options.
Raschig process: The Raschig-Hooker process is a process mainly used for the production of phenol. However, the main step in the production of phenol by this process is the production of chlorobenzene from benzene.
Wurtz-Fittig reaction: It is the chemical reaction of aryl halides with alkyl halides to produce substituted aromatic compounds in the presence of sodium metal and ether.
Friedel-Crafts reaction: These are a set of reactions developed in the year 1877 in order to attach substituents to an aromatic ring. There are two broad classifications namely, alkylation and acylation reactions.
Grignard reaction: It is an organometallic chemical reaction in which alkyl, allyl, vinyl halides are added to carbonyl compounds. This reaction is mainly used for the formation of new carbon-carbon bonds.
From the above evaluation we can conclude that chlorobenzene is prepared commercially by the Raschig process.
Therefore, the correct answer is option (A).
Note: It is important to know that chlorobenzene can be prepared from various set of reactions like the Gattermann and Sandmeyer reaction. However, these reactions are not feasible on a commercial scale. Thus, Raschig process is preferred for the production of chlorobenzene in bulk quantity.
Complete answer:
The expanded structure for chlorobenzene is :

We will now evaluate the processes mentioned in the options.
Raschig process: The Raschig-Hooker process is a process mainly used for the production of phenol. However, the main step in the production of phenol by this process is the production of chlorobenzene from benzene.
Wurtz-Fittig reaction: It is the chemical reaction of aryl halides with alkyl halides to produce substituted aromatic compounds in the presence of sodium metal and ether.
Friedel-Crafts reaction: These are a set of reactions developed in the year 1877 in order to attach substituents to an aromatic ring. There are two broad classifications namely, alkylation and acylation reactions.
Grignard reaction: It is an organometallic chemical reaction in which alkyl, allyl, vinyl halides are added to carbonyl compounds. This reaction is mainly used for the formation of new carbon-carbon bonds.
From the above evaluation we can conclude that chlorobenzene is prepared commercially by the Raschig process.
Therefore, the correct answer is option (A).
Note: It is important to know that chlorobenzene can be prepared from various set of reactions like the Gattermann and Sandmeyer reaction. However, these reactions are not feasible on a commercial scale. Thus, Raschig process is preferred for the production of chlorobenzene in bulk quantity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




