
Chlorobenzene can be prepared by reacting aniline with:
(A)- Hydrochloric acid
(B)- Cuprous chloride
(C)- Chlorine in the presence of anhydrous aluminium chloride
(D)- Nitrous acid followed by heating with cuprous chloride
Answer
573.9k+ views
Hint: Start the solution by drawing the structure of the reactant. Recall what type of reactions aniline undergoes with the given reagents in the options. The product formed must not contain any substituent on the benzene ring except chlorine atom.
Complete answer:
-Aniline is a base which is an aromatic amine.
-The question says that aniline is reacted with a reagent and gives chlorobenzene. This means that the amine group of the aniline is to be replaced on reaction with the reagent with a chlorine atom.
-Recall the knowledge that amine group can only be replaced after it has been converted to diazonium salt form which is an unstable compound and direct halogenation of aniline gives halogen derivatives of aniline.
-When hydrochloric acid is added to aniline, the aniline being a base will abstract the proton forming anilinium chloride salt.
-Nitrous acid produced in situ by sodium nitrite and hydrochloric acid on reaction with aniline at low temperature $(0{}^\circ C-5{}^\circ C)$ converts aniline to a benzene diazonium salt and this reaction is known as diazotization.
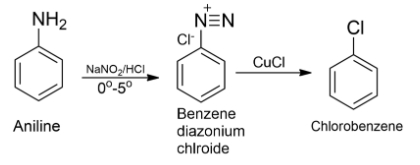
-When a freshly prepared solution of benzene diazonium salt is treated with cuprous oxide, chlorobenzene is obtained. This reaction is known as Sandmeyer’s reaction.
-Therefore, as the conclusion, we can say that chlorobenzene can be prepared by reacting aniline with nitrous acid followed by heating with cuprous chloride (CuCl).
Hence, the correct answer is option D.
Note:
Chlorobenzene can also be prepared by treating diazonium salt with hydrochloric acid and copper powder. This reaction is known as Gattermann reaction and gives a better yield than Sandmeyer reaction. Always remember that aromatic compounds readily undergo electrophilic substitution reaction, therefore direct halogenations will yield halo-substituted compounds.
Complete answer:
-Aniline is a base which is an aromatic amine.
-The question says that aniline is reacted with a reagent and gives chlorobenzene. This means that the amine group of the aniline is to be replaced on reaction with the reagent with a chlorine atom.
-Recall the knowledge that amine group can only be replaced after it has been converted to diazonium salt form which is an unstable compound and direct halogenation of aniline gives halogen derivatives of aniline.
-When hydrochloric acid is added to aniline, the aniline being a base will abstract the proton forming anilinium chloride salt.
-Nitrous acid produced in situ by sodium nitrite and hydrochloric acid on reaction with aniline at low temperature $(0{}^\circ C-5{}^\circ C)$ converts aniline to a benzene diazonium salt and this reaction is known as diazotization.

-When a freshly prepared solution of benzene diazonium salt is treated with cuprous oxide, chlorobenzene is obtained. This reaction is known as Sandmeyer’s reaction.
-Therefore, as the conclusion, we can say that chlorobenzene can be prepared by reacting aniline with nitrous acid followed by heating with cuprous chloride (CuCl).
Hence, the correct answer is option D.
Note:
Chlorobenzene can also be prepared by treating diazonium salt with hydrochloric acid and copper powder. This reaction is known as Gattermann reaction and gives a better yield than Sandmeyer reaction. Always remember that aromatic compounds readily undergo electrophilic substitution reaction, therefore direct halogenations will yield halo-substituted compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




