
Chlorine can be manufactured from
(A) Electrolysis of $NaCl$
(B) Electrolysis of brine
(C) Electrolysis of bleaching powder
(D) All of these
Answer
358.8k+ views
Hint: The process in which a chemical change is produced by passing an electric current through a substance is known as electrolysis. In this process, the cation gets reduced at the cathode and the anion gets oxidised at the anode. A salt bridge containing an electrolyte is used between a cathode and an anode.
Complete Step by Step Solution:
When electrolysis of brine solution takes place, it results in the formation of hydrogen at the cathode and chlorine at the anode. A brine solution is a highly concentrated solution of $NaCl$in water. It can be used as a food preservative, for water treatment, and for industrial refrigeration.
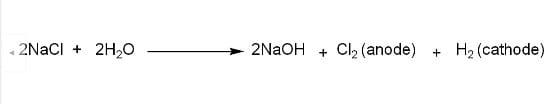
Correct Option: (B) Electrolysis of brine
Additional Information: The process of electrolysis has many applications. Electrolysis is used in metal purification, the production of pure gases, electroplating for corrosion prevention, alkali and alkaline earth metal metallurgy, and so on. Electrolysis depends on the nature of electrodes, electrolytes, and the ions present in the electrolyte. The products of electrolysis are different depending upon the concentration of ions in the solution.
Note: Cell potential is the potential at which the process of electrolysis occurs. It is also known as cell voltage or decomposition voltage. The cell potential may also be defined as the potential difference between two half-cells in an electrochemical series.
Complete Step by Step Solution:
When electrolysis of brine solution takes place, it results in the formation of hydrogen at the cathode and chlorine at the anode. A brine solution is a highly concentrated solution of $NaCl$in water. It can be used as a food preservative, for water treatment, and for industrial refrigeration.

Correct Option: (B) Electrolysis of brine
Additional Information: The process of electrolysis has many applications. Electrolysis is used in metal purification, the production of pure gases, electroplating for corrosion prevention, alkali and alkaline earth metal metallurgy, and so on. Electrolysis depends on the nature of electrodes, electrolytes, and the ions present in the electrolyte. The products of electrolysis are different depending upon the concentration of ions in the solution.
Note: Cell potential is the potential at which the process of electrolysis occurs. It is also known as cell voltage or decomposition voltage. The cell potential may also be defined as the potential difference between two half-cells in an electrochemical series.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

What is the median of the first 10 natural numbers class 10 maths CBSE




