
$C{H_3}CHO$ and $C{H_3}COC{H_3}$ can not be extinguished by:
A.Fehling solution
B.Grignard reagent
C.Schiff’s reagent
D.Tollen’s reagent
Answer
585k+ views
Hint:We know aldehydes and ketones are organic compounds that contain carbonyl groups. We can write the general formula of aldehydes as \[R - CHO\], here R is the alkyl group. We can write the general formula of ketones as $R - CO - R'$, here R and R’ is the alkyl group.
Complete step by step answer:
Aldehydes and ketones contain the carbonyl group.
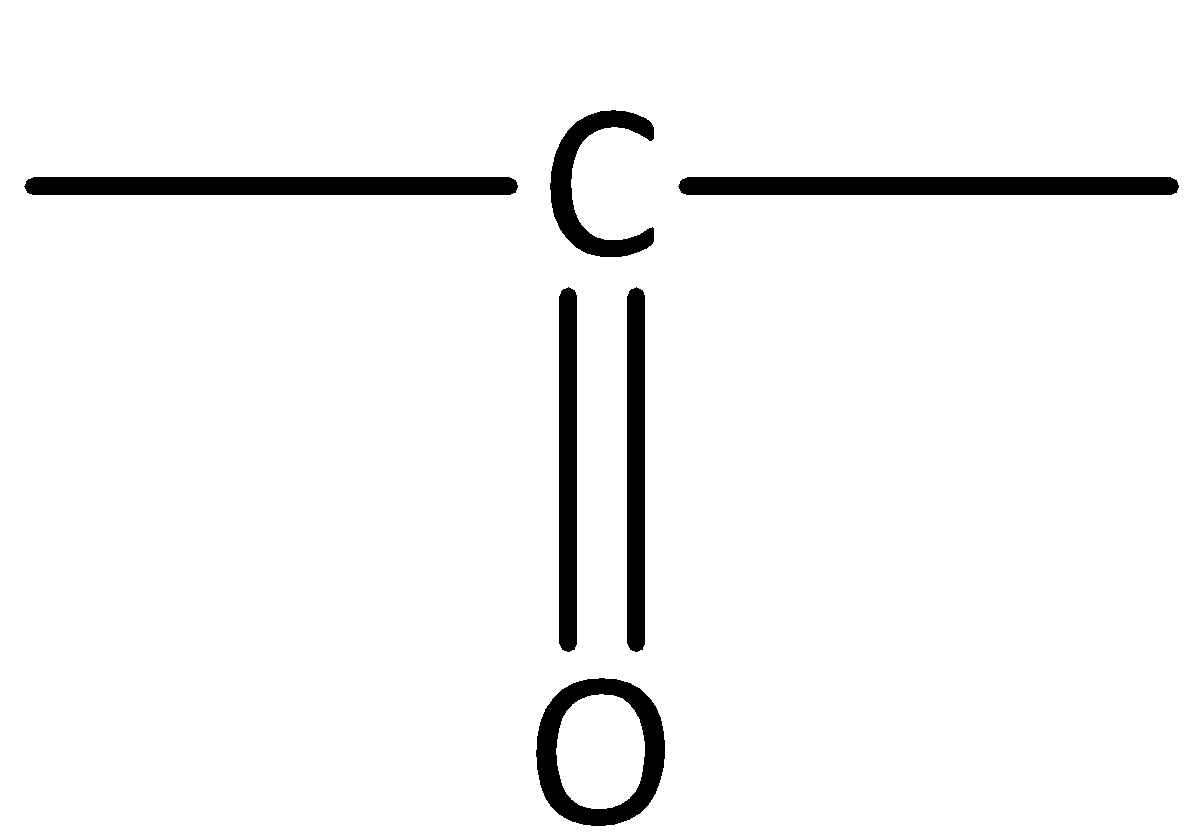
In aldehydes, the carbonyl group is attached at the end of the hydrocarbon chain. The carbonyl carbon is bonded to at least one hydrogen.
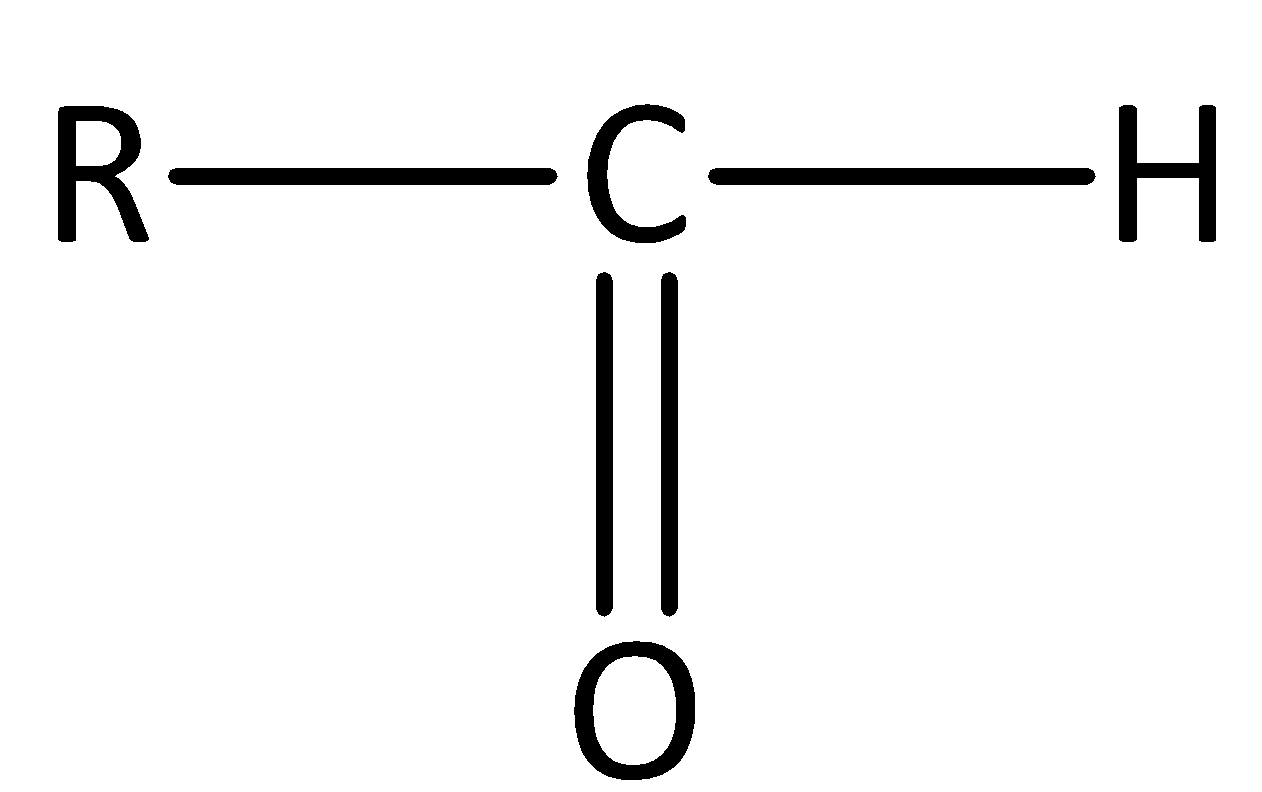
Aldehydes have a general formula of $R - CHO$. The general structure of an aldehyde is,
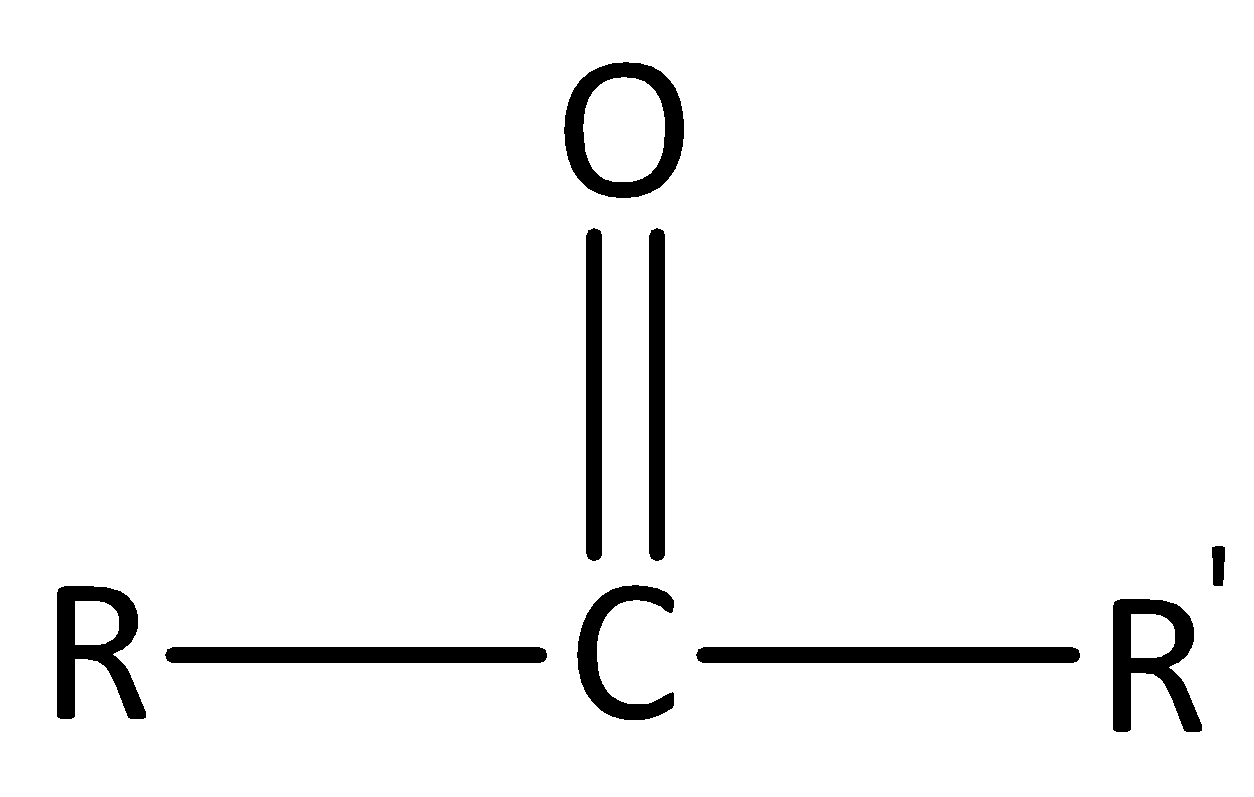
In ketones, the carbonyl group is attached to two carbon atoms. The general formula of a ketone is $R - CO - R'$. The general structure of ketone is,
$C{H_3}CHO$ is an aldehyde and $C{H_3}COC{H_3}$ is a ketone.
Fehling solution is used to differentiate aldehydes and ketones. The presence of brick red precipitate shows the presence of aldehyde (Fehling's test).
Therefore, Option (A) is incorrect.
Tollen’s reagent is used to differentiate aldehydes and ketones. Appearance of a silver mirror along the sides of the test tube indicates the presence of aldehyde (Tollen’s test).
Therefore, option (D) is incorrect.
Aldehyde/Ketone can be treated with 2-3 drops of Schiff’s base. The presence of pink color indicates the presence of aldehyde.
Therefore, option (C) is incorrect.
So, Schiff’s base, Tollen’s reagent and Fehling solution could differentiate $C{H_3}CHO$ and $C{H_3}COC{H_3}$.
Both aldehydes and ketones react with Grignard reagent.
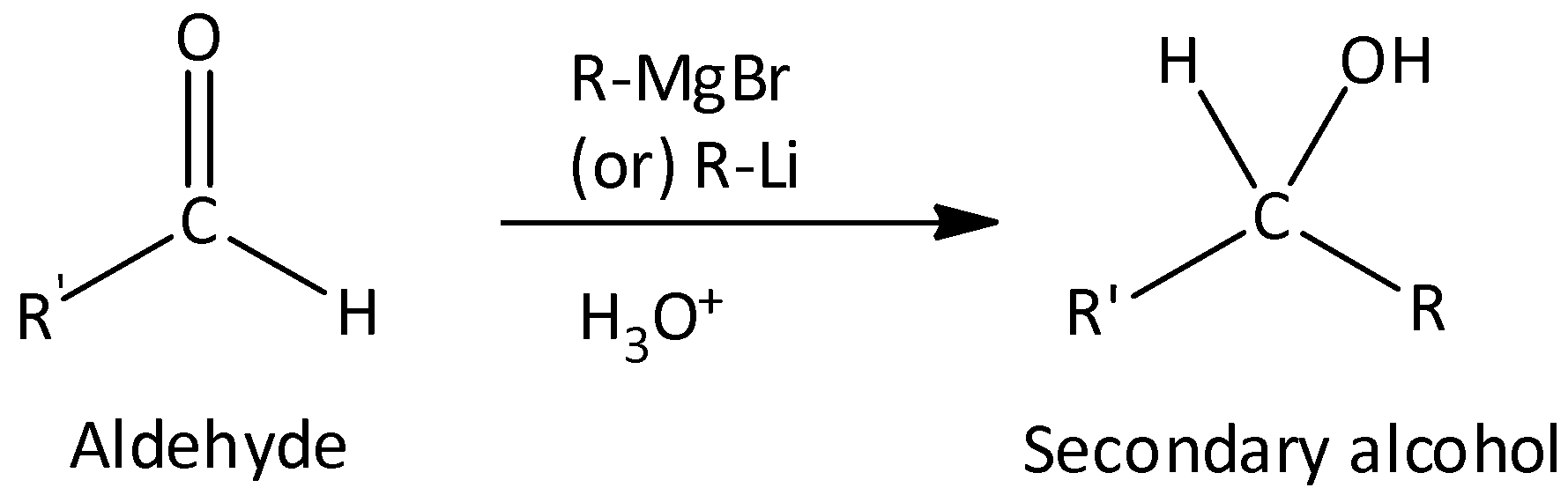
Grignard reagent with aldehydes gives secondary alcohol.
Grignard reagent with ketones gives tertiary alcohol.
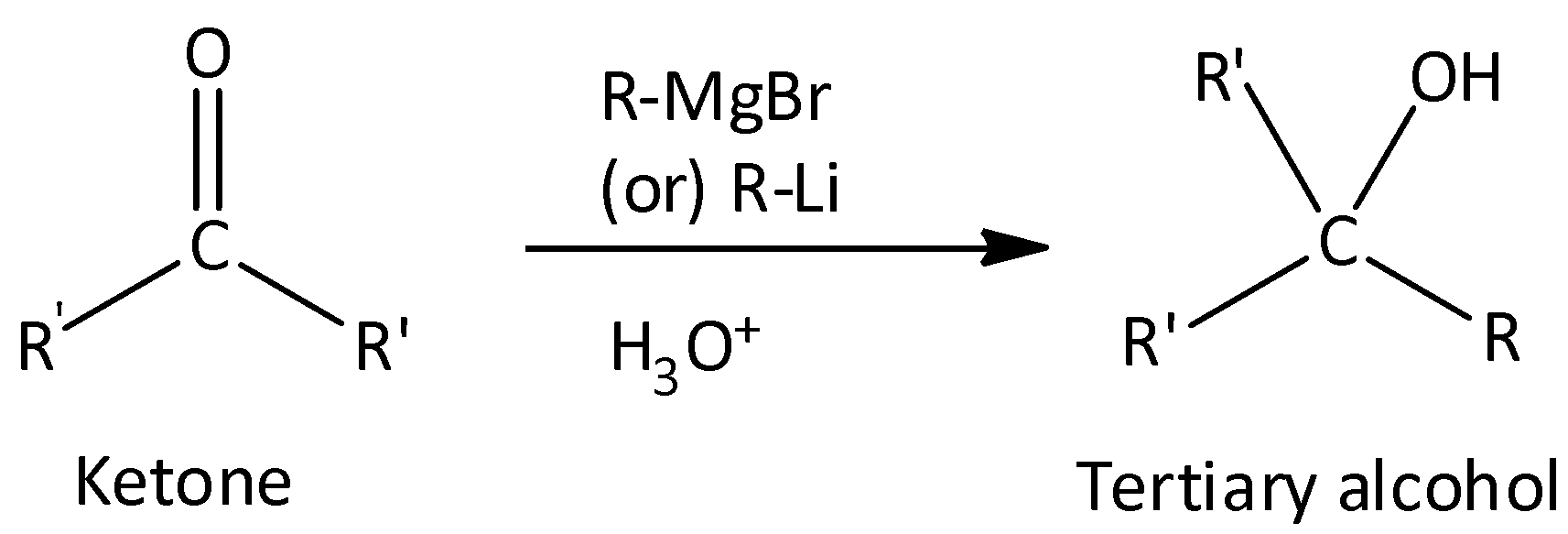
$C{H_3}CHO$ and $C{H_3}COC{H_3}$ cannot be extinguished with Grignard reagent.
Therefore, the option B is correct.
Note:
We also know that aldehydes and ketones cannot form hydrogen bonds to one another, but they can form intermolecular hydrogen bonds with water. As result, the smaller members (five or fewer carbon atoms) are soluble in water. Aldehydes and ketones have lower boiling points when compared to carboxylic acid, representing the presence of weak intermolecular dipole-dipole forces. They do not form hydrogen bonds with other aldehydes or ketones because there is no oxygen-hydrogen bond in the carbonyl group. Due to weaker intermolecular hydrogen bonding in aldehydes and weaker dipole-dipole attractions, they have lower boiling points compared to carboxylic acids.
Complete step by step answer:
Aldehydes and ketones contain the carbonyl group.

In aldehydes, the carbonyl group is attached at the end of the hydrocarbon chain. The carbonyl carbon is bonded to at least one hydrogen.
Aldehydes have a general formula of $R - CHO$. The general structure of an aldehyde is,

In ketones, the carbonyl group is attached to two carbon atoms. The general formula of a ketone is $R - CO - R'$. The general structure of ketone is,

$C{H_3}CHO$ is an aldehyde and $C{H_3}COC{H_3}$ is a ketone.
Fehling solution is used to differentiate aldehydes and ketones. The presence of brick red precipitate shows the presence of aldehyde (Fehling's test).
Therefore, Option (A) is incorrect.
Tollen’s reagent is used to differentiate aldehydes and ketones. Appearance of a silver mirror along the sides of the test tube indicates the presence of aldehyde (Tollen’s test).
Therefore, option (D) is incorrect.
Aldehyde/Ketone can be treated with 2-3 drops of Schiff’s base. The presence of pink color indicates the presence of aldehyde.
Therefore, option (C) is incorrect.
So, Schiff’s base, Tollen’s reagent and Fehling solution could differentiate $C{H_3}CHO$ and $C{H_3}COC{H_3}$.
Both aldehydes and ketones react with Grignard reagent.
Grignard reagent with aldehydes gives secondary alcohol.

Grignard reagent with ketones gives tertiary alcohol.

$C{H_3}CHO$ and $C{H_3}COC{H_3}$ cannot be extinguished with Grignard reagent.
Therefore, the option B is correct.
Note:
We also know that aldehydes and ketones cannot form hydrogen bonds to one another, but they can form intermolecular hydrogen bonds with water. As result, the smaller members (five or fewer carbon atoms) are soluble in water. Aldehydes and ketones have lower boiling points when compared to carboxylic acid, representing the presence of weak intermolecular dipole-dipole forces. They do not form hydrogen bonds with other aldehydes or ketones because there is no oxygen-hydrogen bond in the carbonyl group. Due to weaker intermolecular hydrogen bonding in aldehydes and weaker dipole-dipole attractions, they have lower boiling points compared to carboxylic acids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




