
Cetyl trimethyl ammonium chloride is which type of detergent?
(A) Cationic
(B) Anionic
(C) Biosoft
(D) Non-ionic
Answer
577.8k+ views
Hint: Detergents are the cleansing agents. They do not lead to formation of scum with salts in hard water and they are generally soluble in water. Depending on the electrical charges associated with the detergents there are mainly three different types of detergents i.e. cationic detergents, anionic detergents and non-ionic detergents.
Complete step by step solution:
Cationic detergents produce electrically positive ions in a solution. They contain a long chain which is responsible for the surface properties of cationic detergents. They are usually the quaternary ammonium salts of amines with the chlorides and bromides. Major example of cationic detergent is Cetyl trimethyl ammonium chloride.
Cetyl trimethyl ammonium chloride is a tropical antiseptic and surfactant. It has a long chain of fatty acids or alcohols such as the stearyl alcohols. This cationic surfactant concentration which is present in the conditioners is generally around 1-2 percent. The alcohol concentration in the Cetyl trimethyl ammonium chloride is equal to or greater to alcohol concentration in the cationic detergents.
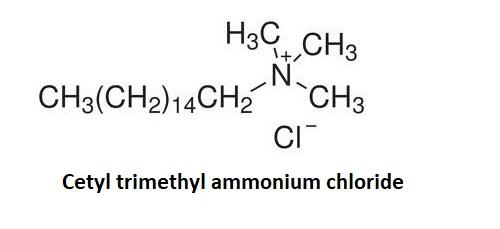
Hence the correct answer is option (A).
Additional information:
-Non-ionic detergents- they do not contain any ions in their constituents. Example of this reagent is stearic acid which reacts with polyethylene glycol.
-Anionic detergents- the anionic detergents have an anionic part which is involved in the cleansing agent. The example of anionic detergents is sodium lauryl sulfate.
-Bio soft detergent is a non ionic surfactant which is an excellent cleaner and it is a very popular detergent. Bio soft detergent is stable in both the solvents i.e. acidic solution and alkaline solution.
Note: Do not get confused between the cationic and the anionic detergents. The cationic detergents contain a hydrophobic component of the quaternary ammonium as the polar end and the ammonium center has a positively charged nitrogen. Whereas in anionic detergents it contains an anion at the soluble end of the long hydrocarbon chain. The cationic detergents have good germicidal properties.
Complete step by step solution:
Cationic detergents produce electrically positive ions in a solution. They contain a long chain which is responsible for the surface properties of cationic detergents. They are usually the quaternary ammonium salts of amines with the chlorides and bromides. Major example of cationic detergent is Cetyl trimethyl ammonium chloride.
Cetyl trimethyl ammonium chloride is a tropical antiseptic and surfactant. It has a long chain of fatty acids or alcohols such as the stearyl alcohols. This cationic surfactant concentration which is present in the conditioners is generally around 1-2 percent. The alcohol concentration in the Cetyl trimethyl ammonium chloride is equal to or greater to alcohol concentration in the cationic detergents.

Hence the correct answer is option (A).
Additional information:
-Non-ionic detergents- they do not contain any ions in their constituents. Example of this reagent is stearic acid which reacts with polyethylene glycol.
-Anionic detergents- the anionic detergents have an anionic part which is involved in the cleansing agent. The example of anionic detergents is sodium lauryl sulfate.
-Bio soft detergent is a non ionic surfactant which is an excellent cleaner and it is a very popular detergent. Bio soft detergent is stable in both the solvents i.e. acidic solution and alkaline solution.
Note: Do not get confused between the cationic and the anionic detergents. The cationic detergents contain a hydrophobic component of the quaternary ammonium as the polar end and the ammonium center has a positively charged nitrogen. Whereas in anionic detergents it contains an anion at the soluble end of the long hydrocarbon chain. The cationic detergents have good germicidal properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




