
Carry out following conversions
a.Benzene to m-Chloro bromobenzene
b.Chloromethane to Ethanoic acid
c.Aniline to p-Chloroaniline
Answer
501.9k+ views
Hint: We will use different types of reaction to carry out the given conversions. In the first conversion we will perform nitration, chlorination hydrogenation, Oxidation, Bromination in order. In the second conversion we will use Grignard agent and Carbon dioxide and then perform hydrolysis. In third conversion we will convert aniline to chlorobenzene then perform nitration and chlorination.
Complete answer:
Let’s see the first conversion
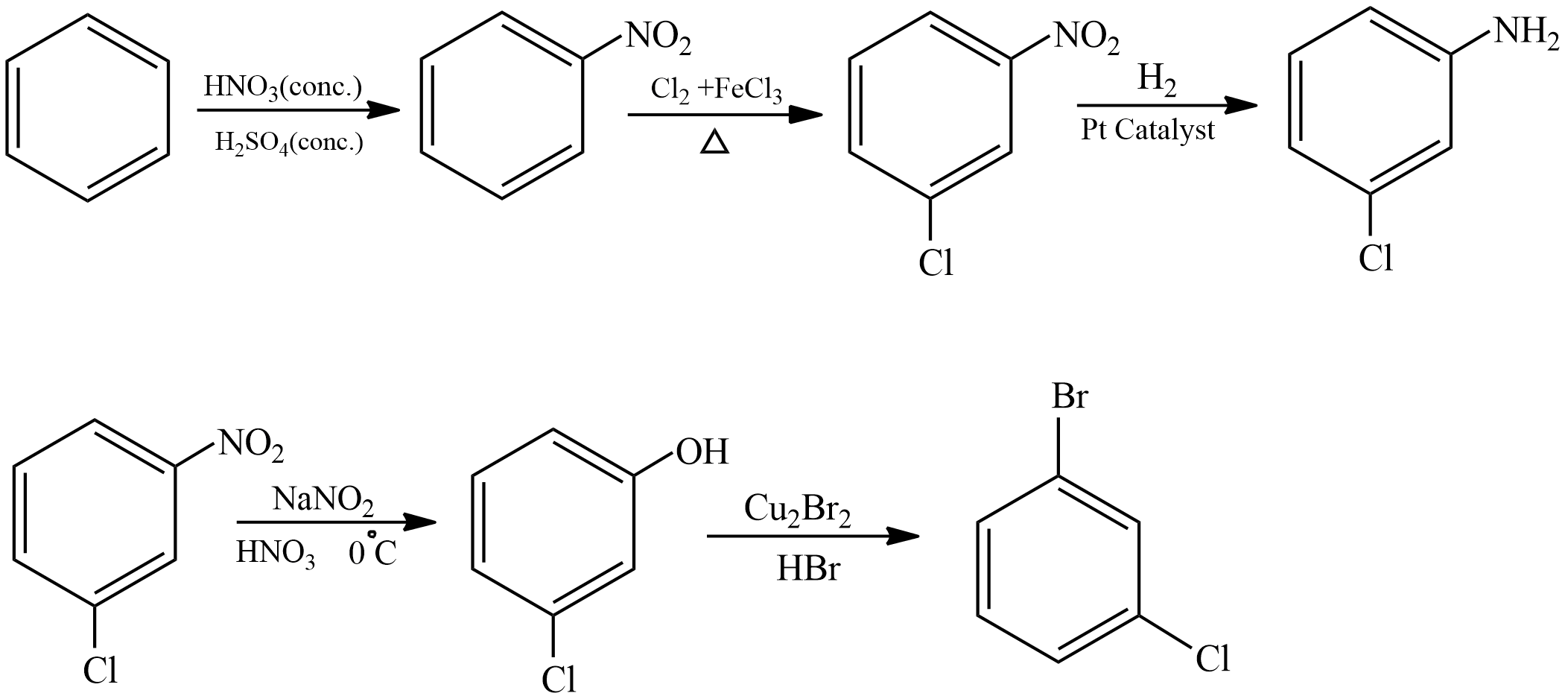
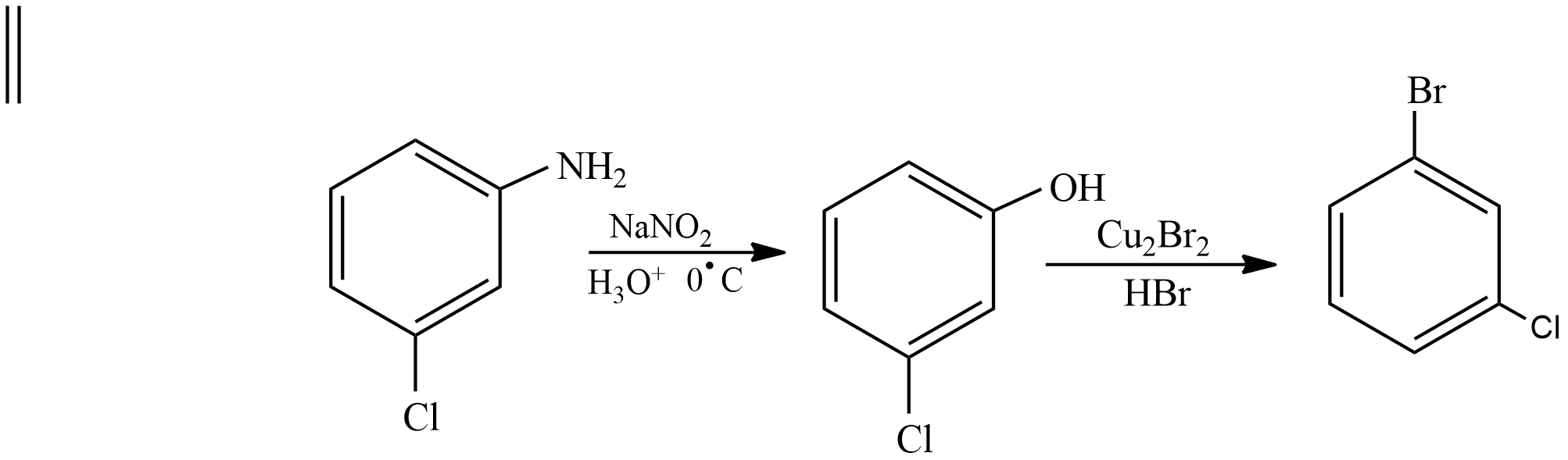
First benzene is treated with concentrated nitric acid and concentrated sulphuric acid to form Nitrobenzene. Then nitrobenzene is heated with Chlorine in presence of Iron (III) chloride as the Nitro group is a meta director Chlorine is attached to meta position. Then m-Nitro chlorobenzene undergoes hydrogenation in presence of platinum catalyst, Nitro group is converted into amine and product formed is known as m-Chloroaniline. Then it undergoes Sandmeyer reaction and an amine group is converted into Hydroxyl group, product formed is known as m-Chlorophenol. Then it undergoes substitution reaction with Hydrogen bromide in presence of copper bromide and Hydroxyl group is substituted with Bromine the product formed is m-Chloro bromobenzene.
Let’s see the second conversion
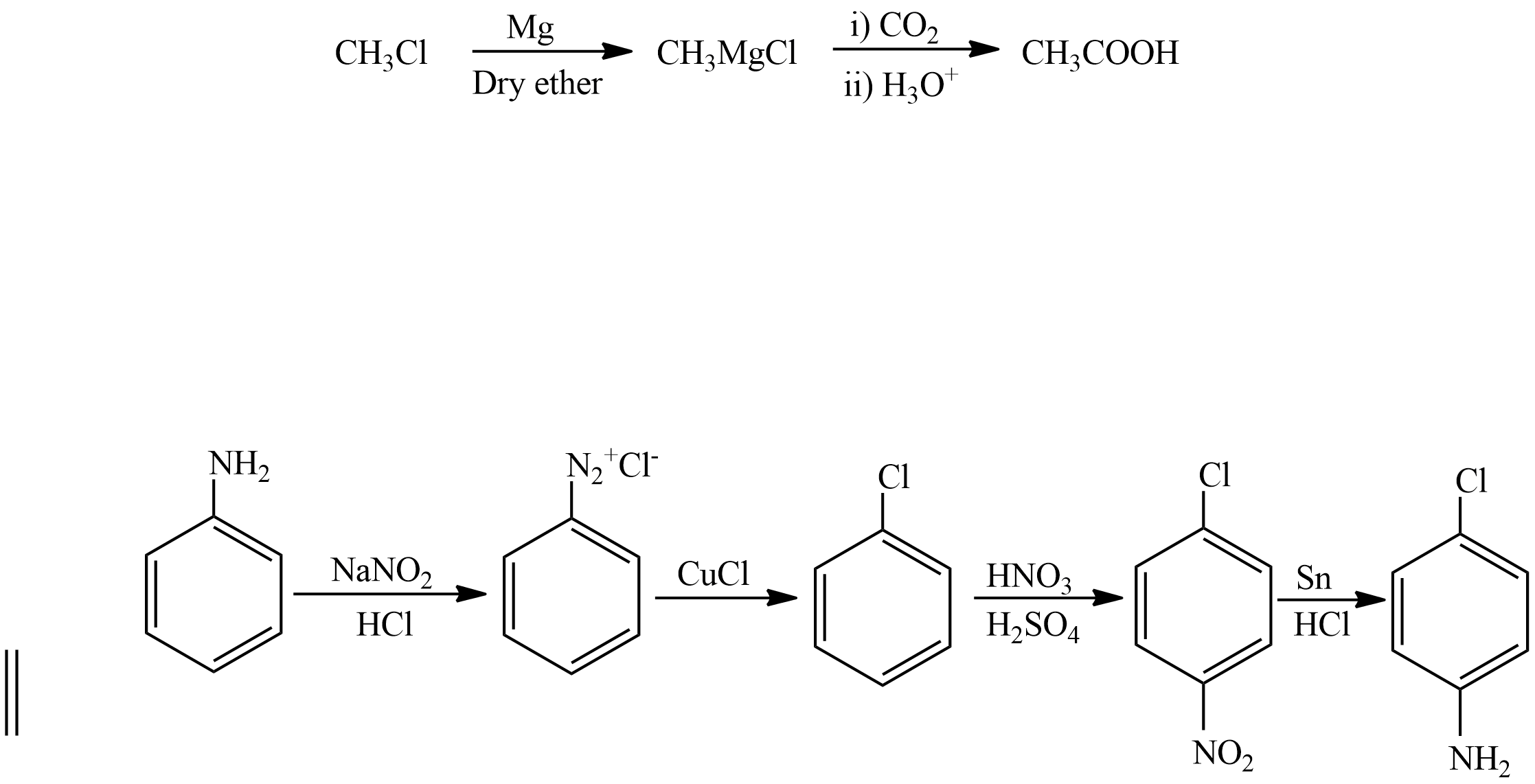
Chloromethane is treated with Magnesium in presence of Dry ether to form a magnesium chloride salt, it is known as Grignard reagent then it is treated with carbon dioxide followed by acidification to form ethanoic acid.
Let’s see the third conversion
Aniline is treated with Sodium nitrite with Hydrochloric acid to form Benzene Diazonium chloride then it is treated with copper chloride to form Chlorobenzene. Chlorobenzene undergoes nitration in presence of Nitric acid and Sulphuric acid, then it undergoes Reduction in presence of Tin and hydrochloric acid to form p-Chloroaniline.
Note:
Grignard reagent on reaction with carbon dioxide gives a product known as carboxylates, when these carboxylates are subjected to acidification in presence of strong acid, they form carboxylic acid. Since the carbon of Carbon dioxide is added, the Carboxylic acid will contain one more carbon than the Grignard reagent.
Complete answer:
Let’s see the first conversion


First benzene is treated with concentrated nitric acid and concentrated sulphuric acid to form Nitrobenzene. Then nitrobenzene is heated with Chlorine in presence of Iron (III) chloride as the Nitro group is a meta director Chlorine is attached to meta position. Then m-Nitro chlorobenzene undergoes hydrogenation in presence of platinum catalyst, Nitro group is converted into amine and product formed is known as m-Chloroaniline. Then it undergoes Sandmeyer reaction and an amine group is converted into Hydroxyl group, product formed is known as m-Chlorophenol. Then it undergoes substitution reaction with Hydrogen bromide in presence of copper bromide and Hydroxyl group is substituted with Bromine the product formed is m-Chloro bromobenzene.
Let’s see the second conversion

Chloromethane is treated with Magnesium in presence of Dry ether to form a magnesium chloride salt, it is known as Grignard reagent then it is treated with carbon dioxide followed by acidification to form ethanoic acid.
Let’s see the third conversion

Aniline is treated with Sodium nitrite with Hydrochloric acid to form Benzene Diazonium chloride then it is treated with copper chloride to form Chlorobenzene. Chlorobenzene undergoes nitration in presence of Nitric acid and Sulphuric acid, then it undergoes Reduction in presence of Tin and hydrochloric acid to form p-Chloroaniline.
Note:
Grignard reagent on reaction with carbon dioxide gives a product known as carboxylates, when these carboxylates are subjected to acidification in presence of strong acid, they form carboxylic acid. Since the carbon of Carbon dioxide is added, the Carboxylic acid will contain one more carbon than the Grignard reagent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




