
Carbon monoxide has a ………. bond between C and O.
(A) Triple
(B) Double
(C) Single
(D) Ionic
Answer
591.9k+ views
Hint: There are two covalent bonds and one coordinate bond present in Carbon monoxide molecules. The bond order of Carbon monoxide is three. So, the bond between C and O is strong enough.
Complete step by step answer:
In CO, there is a triple bond between C and O.
Out of these three bonds, two are formed by sharing of electrons (covalent bond) between C and O. And one bond is a coordination bond, in which the Oxygen donated its lone pair of electrons to the Carbon atom.
Structure of Carbon monoxide is given below:
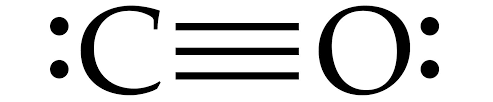
Carbon monoxide is linear in structure , with a bond angle of ${ 180 }^{ 0 }$ in between C and O atoms.
As we know that one of the bonds in CO is a coordination bond, which is formed by the donation of lone pairs of electrons from Oxygen to Carbon atoms.
We should know one more thing here that, since the electrons are being donated from Oxygen atom to Carbon atom, therefore, in actual structure of Carbon monoxide, there exists a partial positive charge on Oxygen atom and Partial negative charge on Carbon atom as shown below:
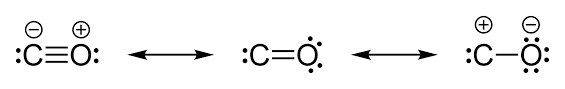
From this, we can get to know that there is a triple bond in between C and O in CO molecules.
Therefore, Option (A) triple bond is the correct answer.
Additional Information: Carbon monoxide is also a well known ${ \pi }$ -acid ligand / ${ \pi }$ acceptor ligand.
A ${ \pi }$ acid ligand is a ligand that can form an additional double bond by accepting a lone pair of electrons from the metal, along with the normal ${ \sigma }$ bonds .
Note: One can also explain this by the bond number of CO molecule, which is determined by the equation $\frac { 1 }{ 2 } \left( { N }_{ b }-{ N }_{ a } \right) $ , where ${ N }_{ b }$ is the number of electrons in the bonding orbital and ${ N }_{ a }$ is the number of electrons in antibonding orbitals. In the case of CO, this value comes out to be 3 which implies that it has a triple bond.
Complete step by step answer:
In CO, there is a triple bond between C and O.
Out of these three bonds, two are formed by sharing of electrons (covalent bond) between C and O. And one bond is a coordination bond, in which the Oxygen donated its lone pair of electrons to the Carbon atom.
Structure of Carbon monoxide is given below:
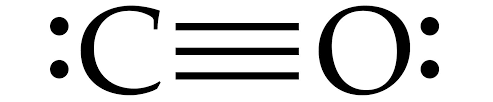
Carbon monoxide is linear in structure , with a bond angle of ${ 180 }^{ 0 }$ in between C and O atoms.
As we know that one of the bonds in CO is a coordination bond, which is formed by the donation of lone pairs of electrons from Oxygen to Carbon atoms.
We should know one more thing here that, since the electrons are being donated from Oxygen atom to Carbon atom, therefore, in actual structure of Carbon monoxide, there exists a partial positive charge on Oxygen atom and Partial negative charge on Carbon atom as shown below:
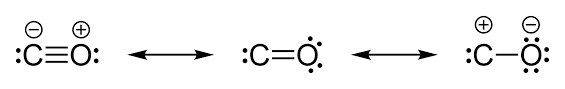
From this, we can get to know that there is a triple bond in between C and O in CO molecules.
Therefore, Option (A) triple bond is the correct answer.
Additional Information: Carbon monoxide is also a well known ${ \pi }$ -acid ligand / ${ \pi }$ acceptor ligand.
A ${ \pi }$ acid ligand is a ligand that can form an additional double bond by accepting a lone pair of electrons from the metal, along with the normal ${ \sigma }$ bonds .
Note: One can also explain this by the bond number of CO molecule, which is determined by the equation $\frac { 1 }{ 2 } \left( { N }_{ b }-{ N }_{ a } \right) $ , where ${ N }_{ b }$ is the number of electrons in the bonding orbital and ${ N }_{ a }$ is the number of electrons in antibonding orbitals. In the case of CO, this value comes out to be 3 which implies that it has a triple bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




