
How many carbocation isomers are possible for the compound with molecular formula ${C_3}H_5^ + $?
Answer
566.7k+ views
Hint: We must remember that the isomers are compounds having the same chemical formula but different chemical structure. Here we have a compound and we need to find the number of isomers that can be formed. The number of carbons is 3, hence the orientation will be more of chain orientation and not stereo type orientation. Stereo type of orientation is possible in high order carbon compounds.
Complete answer:
Given compound - ${C_3}H_5^ + $
To determine the number of isomers, we will need to draw the chemical orientation of the given compound.
We can draw the possible orientation for this given compound as,
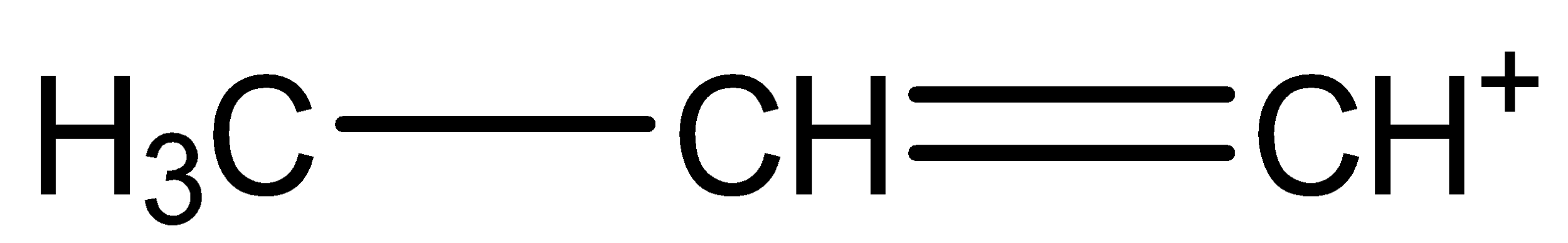
(a)
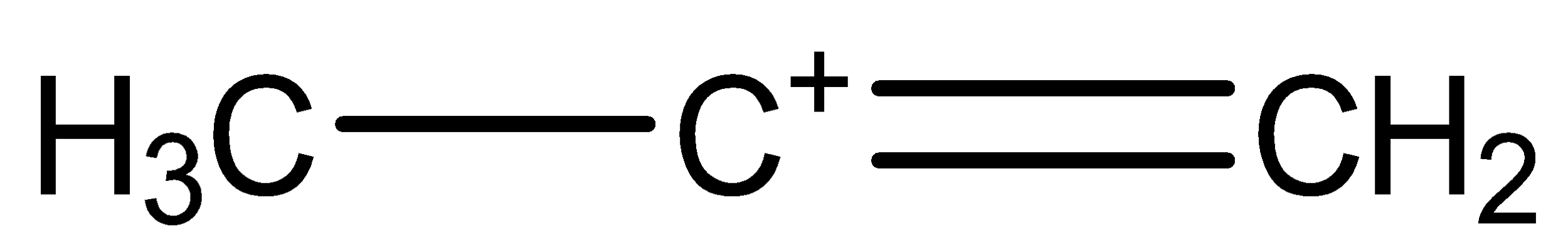
(b)
Here, two orientations are possible as shown in chemical structure (a) and (b).
The (a) chemical structure is called n-propene carbocation.
The (b) chemical structure is called secondary-propene carbocation.
The bonding between the three carbon atoms is one with single bond and one with double bond.
The Hydrogen atoms are arranged by choosing different orientation, keeping one unbonded arm, thus forming a cation.
Hence, the correct answer is 2.
Only 2 isomers are possible for the given carbocation - ${C_3}H_5^ + $.
Note:
As we know that the isomers are the different structures of the same chemical formula. We must remember that determining the number of isomers that can be formed can be quite tricky at times, especially when the order of carbon atoms is high. But for a lower order carbon compound which is given here. It is quite easy to determine by drawing the different possible orientations.
Complete answer:
Given compound - ${C_3}H_5^ + $
To determine the number of isomers, we will need to draw the chemical orientation of the given compound.
We can draw the possible orientation for this given compound as,

(a)
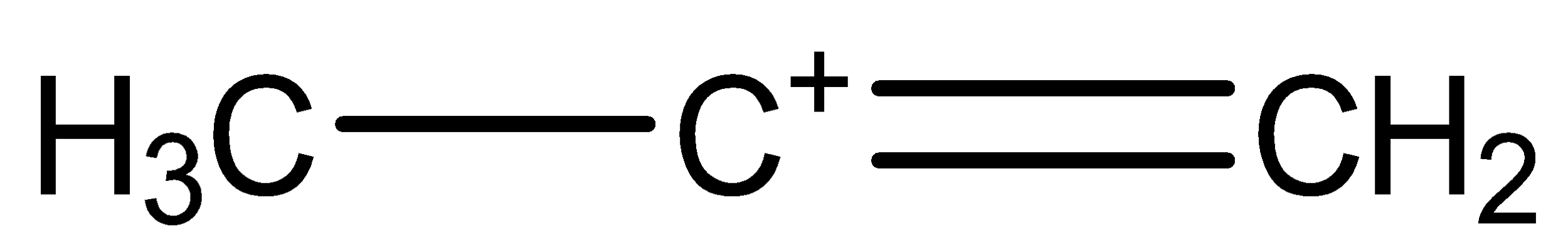
(b)
Here, two orientations are possible as shown in chemical structure (a) and (b).
The (a) chemical structure is called n-propene carbocation.
The (b) chemical structure is called secondary-propene carbocation.
The bonding between the three carbon atoms is one with single bond and one with double bond.
The Hydrogen atoms are arranged by choosing different orientation, keeping one unbonded arm, thus forming a cation.
Hence, the correct answer is 2.
Only 2 isomers are possible for the given carbocation - ${C_3}H_5^ + $.
Note:
As we know that the isomers are the different structures of the same chemical formula. We must remember that determining the number of isomers that can be formed can be quite tricky at times, especially when the order of carbon atoms is high. But for a lower order carbon compound which is given here. It is quite easy to determine by drawing the different possible orientations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




