
Can you make isomers of propane?
a.) True
b.) False
Answer
600.3k+ views
Hint: We should know about the structure of propane before answering this question. Propane is a molecule that contains three carbon atoms. The three carbon bonds together through single bonds.
Step by step answer:
The structure of Propane is given below. This will help in the better understanding of the compound.
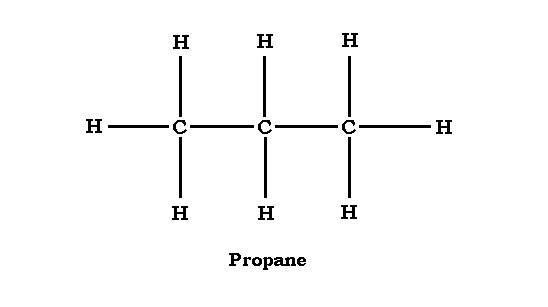
From the structure we can say that each carbon molecule must have four bonds. Therefore, the centre carbon, which is already pointed to, has carbon bonds to hydrogen. The outer carbons, which of them are already bonded to one carbon, each bonds to three hydrogen.
Because hydrogen can form one bond, the hydrogen can not be placed between two carbon atoms. They must be on the outside of the carbon atoms.
Therefore, we can say that propane has no isomers. There is only one possible structure of propane, which is shown in the above diagram.
So we can say that due to the insufficient number of carbon atoms in propane, it is not possible for propane to exist in a branched isomer state. So the answer to the question that Can you make isomers of propane, is false. Option B is the correct answer.
Additional Information:
Isomerism is defined as the phenomenon in which more than one compounds have the same chemical formula. But the main fact is, the chemical structures of the compounds are different.
Note: The interesting fact about propane that we should know is that propane is transported and stored as liquid propane, which is sometimes also known as LPG or LP gas. It is a colorless and odorless gas. As propane is odorless, it is intentionally odorized so that it can be detected. The odour is similar to rotten eggs.
Step by step answer:
The structure of Propane is given below. This will help in the better understanding of the compound.

From the structure we can say that each carbon molecule must have four bonds. Therefore, the centre carbon, which is already pointed to, has carbon bonds to hydrogen. The outer carbons, which of them are already bonded to one carbon, each bonds to three hydrogen.
Because hydrogen can form one bond, the hydrogen can not be placed between two carbon atoms. They must be on the outside of the carbon atoms.
Therefore, we can say that propane has no isomers. There is only one possible structure of propane, which is shown in the above diagram.
So we can say that due to the insufficient number of carbon atoms in propane, it is not possible for propane to exist in a branched isomer state. So the answer to the question that Can you make isomers of propane, is false. Option B is the correct answer.
Additional Information:
Isomerism is defined as the phenomenon in which more than one compounds have the same chemical formula. But the main fact is, the chemical structures of the compounds are different.
Note: The interesting fact about propane that we should know is that propane is transported and stored as liquid propane, which is sometimes also known as LPG or LP gas. It is a colorless and odorless gas. As propane is odorless, it is intentionally odorized so that it can be detected. The odour is similar to rotten eggs.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




