
Calculate the work done by adiabatic compression of one mole of an ideal gas (mono atomic) from an initial pressure of 1 atm to final pressure of 2 atm. Initial temperature = 300 K.
(a)If the process is carried out reversibly
(b) If the process is carried out irreversibly against 2 atm external pressure.
Compute the final volume reached by gas in two cases and describe the work graphically.
Answer
531.6k+ views
Hint: Adiabatic compression is taking place, means that the energy is transferred to the surroundings. Here compression is taking place that causes a temperature rise in the gas.
Formula used: ${{W}_{adiabatic}}=\int{-PdV}$
Complete answer:
(a) We have been given 1 mole of an ideal gas with initial pressure ${{P}_{1}}$ = 1 atm, final pressure${{P}_{2}}$= 2 atm. With initial temperature= 300 K. Heat capacity of an ideal gas at constant volume ${{C}_{v}}=$ 3 $\dfrac{3R}{2}$ , so, r= $\dfrac{5}{3}$
Now to calculate work done , when the process is carried reversibly, we have to take out the volume,
As, PV=nRT
We have, P = ${{P}_{2}}-{{P}_{1}}$= 1 , n = 1, R= 0.082, T= 300 K
So, $1\times V=1\times 0.082\times 300$
${{V}_{1}}$ = 24.63
For reversible process in adiabatic compression, ${{P}_{1}}{{V}_{1}}={{P}_{2}}{{V}_{2}}$
$1\times {{(24.63)}^{r}}=2\times {{V}_{2}}^{r}$where r = 1.66, so, ${{\left( \dfrac{24.6}{{{V}_{2}}} \right)}^{1.66}}$
Therefore, $1.66\times \log \left( \dfrac{24.6}{{{V}_{2}}} \right)=\log \,2$
So, ${{V}_{2}}=18.15\,L$
Now, in adiabatic compression work done is, ${{W}_{adiabatic}}=\int{-PdV}$
${{W}_{adiabatic}}=\int{\dfrac{CdV}{{{V}^{1-r}}}}$= 1194.72 J
So, work done is 1194.72 J
Now, for final temperature,${{P}^{1-r}}{{V}^{r}}$ = constant for reversible compression,
${{P}_{2}}^{1-\dfrac{5}{3}}{{T}_{2}}^{\dfrac{5}{3}}={{P}_{1}}^{-\dfrac{2}{3}}{{T}_{1}}^{3}$
${{2}^{-2300}}{{\left[ \dfrac{T}{300} \right]}^{5}}=1$
$\dfrac{T}{300}={{4}^{\dfrac{1}{5}}}$
T = ${{4}^{{}^{1}/{}_{5}}}\times $ 300
${{T}_{2}}$ = 395.85
Hence, work done in this case is 1194.72 J and final volume is ${{V}_{2}}=18.15\,L$
(b) now, we have to calculate work done when the process is irreversible against 2 atm external pressure.
For this, PV=nRT = 300 R
We know that, $n{{C}_{v}}\Delta T=-{{P}_{2}}({{V}_{2}}-{{V}_{1}})$
$1\times \dfrac{3R}{2}({{T}_{2}}-{{T}_{1}})=-2({{V}_{2}}-{{V}_{1}})$
$\dfrac{3R}{2}({{T}_{2}}-300)=-{{V}_{2}}\times R+2\times 300\,R$
$\dfrac{3R}{2}-450=-{{T}_{2}}.1+600\,$
$\dfrac{5{{T}_{2}}}{2}=1050$
${{T}_{2}}=\dfrac{2100}{5}$
${{T}_{2}}=420K$
So, volume will be, ${{V}_{2}}=\dfrac{R\times 420}{2}$ = 210 R
${{V}_{2}}=$ 17.24 L
So, work done will be $W=-2({{V}_{2}}-{{V}_{1}})$
$W=-2(210R-300R)$
W = 180 R
W = 1496.525 J
Hence, work done in this case is 1496.525 J and final volume is ${{V}_{2}}=$ 17.24 L
For both the volumes the graph between pressure and volume will be plotted as,
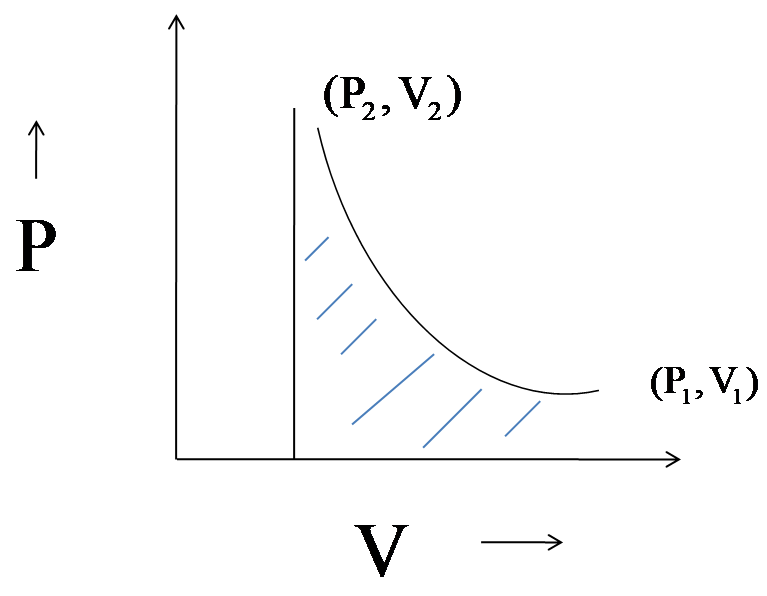
The work done in adiabatic compression is the area under the curve. In both the cases, the final volume is greater than the initial volume.
Note:
In adiabatic compression we will obtain the final temperatures in both the cases to be greater than initial temperatures, as the gas has been compressed. In the second case, the final temperature is higher than the first case, because external pressure of 2 atm is also applied.
Formula used: ${{W}_{adiabatic}}=\int{-PdV}$
Complete answer:
(a) We have been given 1 mole of an ideal gas with initial pressure ${{P}_{1}}$ = 1 atm, final pressure${{P}_{2}}$= 2 atm. With initial temperature= 300 K. Heat capacity of an ideal gas at constant volume ${{C}_{v}}=$ 3 $\dfrac{3R}{2}$ , so, r= $\dfrac{5}{3}$
Now to calculate work done , when the process is carried reversibly, we have to take out the volume,
As, PV=nRT
We have, P = ${{P}_{2}}-{{P}_{1}}$= 1 , n = 1, R= 0.082, T= 300 K
So, $1\times V=1\times 0.082\times 300$
${{V}_{1}}$ = 24.63
For reversible process in adiabatic compression, ${{P}_{1}}{{V}_{1}}={{P}_{2}}{{V}_{2}}$
$1\times {{(24.63)}^{r}}=2\times {{V}_{2}}^{r}$where r = 1.66, so, ${{\left( \dfrac{24.6}{{{V}_{2}}} \right)}^{1.66}}$
Therefore, $1.66\times \log \left( \dfrac{24.6}{{{V}_{2}}} \right)=\log \,2$
So, ${{V}_{2}}=18.15\,L$
Now, in adiabatic compression work done is, ${{W}_{adiabatic}}=\int{-PdV}$
${{W}_{adiabatic}}=\int{\dfrac{CdV}{{{V}^{1-r}}}}$= 1194.72 J
So, work done is 1194.72 J
Now, for final temperature,${{P}^{1-r}}{{V}^{r}}$ = constant for reversible compression,
${{P}_{2}}^{1-\dfrac{5}{3}}{{T}_{2}}^{\dfrac{5}{3}}={{P}_{1}}^{-\dfrac{2}{3}}{{T}_{1}}^{3}$
${{2}^{-2300}}{{\left[ \dfrac{T}{300} \right]}^{5}}=1$
$\dfrac{T}{300}={{4}^{\dfrac{1}{5}}}$
T = ${{4}^{{}^{1}/{}_{5}}}\times $ 300
${{T}_{2}}$ = 395.85
Hence, work done in this case is 1194.72 J and final volume is ${{V}_{2}}=18.15\,L$
(b) now, we have to calculate work done when the process is irreversible against 2 atm external pressure.
For this, PV=nRT = 300 R
We know that, $n{{C}_{v}}\Delta T=-{{P}_{2}}({{V}_{2}}-{{V}_{1}})$
$1\times \dfrac{3R}{2}({{T}_{2}}-{{T}_{1}})=-2({{V}_{2}}-{{V}_{1}})$
$\dfrac{3R}{2}({{T}_{2}}-300)=-{{V}_{2}}\times R+2\times 300\,R$
$\dfrac{3R}{2}-450=-{{T}_{2}}.1+600\,$
$\dfrac{5{{T}_{2}}}{2}=1050$
${{T}_{2}}=\dfrac{2100}{5}$
${{T}_{2}}=420K$
So, volume will be, ${{V}_{2}}=\dfrac{R\times 420}{2}$ = 210 R
${{V}_{2}}=$ 17.24 L
So, work done will be $W=-2({{V}_{2}}-{{V}_{1}})$
$W=-2(210R-300R)$
W = 180 R
W = 1496.525 J
Hence, work done in this case is 1496.525 J and final volume is ${{V}_{2}}=$ 17.24 L
For both the volumes the graph between pressure and volume will be plotted as,

The work done in adiabatic compression is the area under the curve. In both the cases, the final volume is greater than the initial volume.
Note:
In adiabatic compression we will obtain the final temperatures in both the cases to be greater than initial temperatures, as the gas has been compressed. In the second case, the final temperature is higher than the first case, because external pressure of 2 atm is also applied.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




