
Calculate the total number of unpaired electrons in \[{\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}\]?
Answer
569.4k+ views
Hint: We have to remember the VBT (Valence Bond Theory), it is useful for explaining the chemical bonding. VBT is based on covalent interactions between the central metal and the ligands. This theory is given by Linus Pauling. This theory also explains the geometry, magnetic behavior and the formation of a complex compound. The name of the compound \[{\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}\] is hexaaquachromium($III$) ion.
Complete step by step answer:
Let us see how the Valence Bond Theory explains the bonding in \[{\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}\], hexaaquachromium($III$) ion.
First of all we know that chromium has an atomic number of \[24\] and outer most electronic configuration is $3{d^5}4{s^1}$, because the half-filled d orbitals have extra stability. So, in chromium, one electron of $4s$ orbital goes to $3d$ orbital and it is extra stable. Hexaaquachromium ($III$) ion coordination number is six, the total number of electron pairs accepted by the central metal atom (chromium) will be equal to six.
The outer most electronic configuration of chromium is $3{d^5}4{s^1}$,
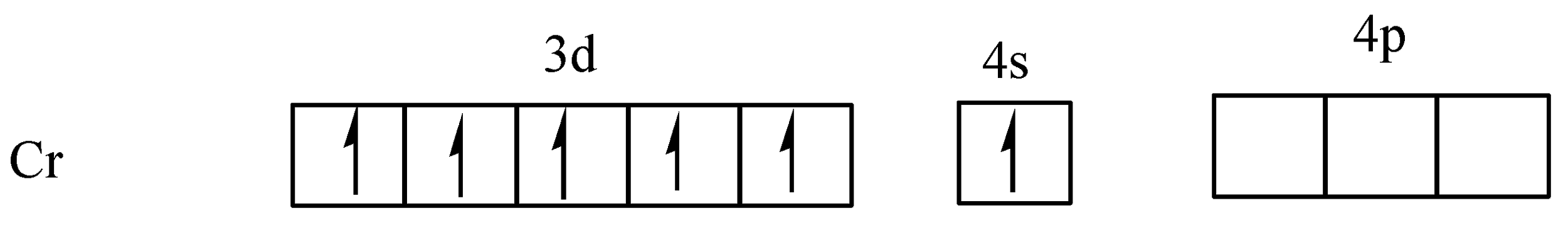
From the above diagram clearly shows that the five orbitals are singly filled. In the complex,\[{\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}\], the ligand ${H_2}O$ is a weak ligand, so no electron pair takes place and chromium has $ + 3$ oxidation state,
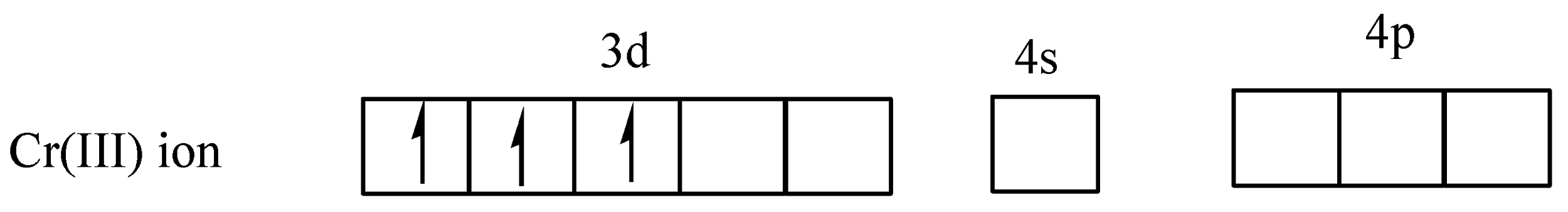
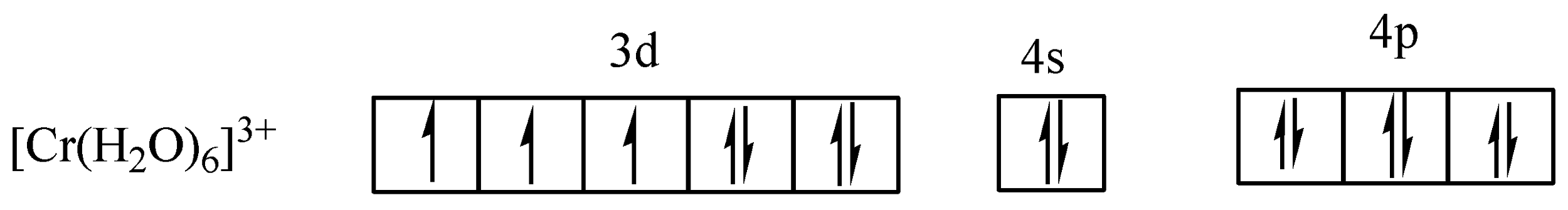
In (c) the arrows ($ \uparrow \downarrow $) represent electron pairs donated by ${H_2}O$.
In (c) the six water molecules forming ${d^2}s{p^3}$ hybridization occur resulting in an octahedral of the complex and indicated the complex is paramagnetic.
From (c), there are three ($3$) unpaired electrons in a hexaaquachromium($III$) ion (\[{\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}\]) complex.
Note: We have to remember that the valence Bond Theory important aspect is the condition of maximum overlap, which leads to the formation of the strongest possible bonds. Some of the ligands are strong ligands (e.g. $C{N^ - }$,$N{O_2}^ - $,$SC{N^ - }$etc.), thus forced pairing will occur and the electrons are paired.
Complete step by step answer:
Let us see how the Valence Bond Theory explains the bonding in \[{\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}\], hexaaquachromium($III$) ion.
First of all we know that chromium has an atomic number of \[24\] and outer most electronic configuration is $3{d^5}4{s^1}$, because the half-filled d orbitals have extra stability. So, in chromium, one electron of $4s$ orbital goes to $3d$ orbital and it is extra stable. Hexaaquachromium ($III$) ion coordination number is six, the total number of electron pairs accepted by the central metal atom (chromium) will be equal to six.
The outer most electronic configuration of chromium is $3{d^5}4{s^1}$,

From the above diagram clearly shows that the five orbitals are singly filled. In the complex,\[{\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}\], the ligand ${H_2}O$ is a weak ligand, so no electron pair takes place and chromium has $ + 3$ oxidation state,


In (c) the arrows ($ \uparrow \downarrow $) represent electron pairs donated by ${H_2}O$.
In (c) the six water molecules forming ${d^2}s{p^3}$ hybridization occur resulting in an octahedral of the complex and indicated the complex is paramagnetic.
From (c), there are three ($3$) unpaired electrons in a hexaaquachromium($III$) ion (\[{\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}\]) complex.
Note: We have to remember that the valence Bond Theory important aspect is the condition of maximum overlap, which leads to the formation of the strongest possible bonds. Some of the ligands are strong ligands (e.g. $C{N^ - }$,$N{O_2}^ - $,$SC{N^ - }$etc.), thus forced pairing will occur and the electrons are paired.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




