
Calculate the oxidation number of sulphur, chromium and nitrogen in ${H_2}S{O_5},C{r_2}O_7^{2 - }$ and $NO_3^ - $ . Suggest structure of these compounds. Count for the fallacy.
Answer
558k+ views
Hint: Oxidation state of an atom is the degree of oxidation of an atom in a compound. The sum of oxidation states of all the atoms is equal to zero or the charge on the whole compound. An element cannot have an oxidation state value exceeding its number of valence electrons. It is considered as a state of fallacy.
Complete step by step answer:
Oxidation state of an atom in a chemical compound is the degree of oxidation of that atom. It is indicated as the charge on an atom when all bonds of that atom are completely ionic with other elements. It is an integer value which may be positive, negative or zero. The oxidation number of a free element is zero and for hydrogen it is equal to +1 but if hydrogen forms a bond with a less electronegative element then its oxidation state is taken as -1. For oxygen oxidation state is taken as $ - 2$ but in case of peroxide it is -1.
For the given question
- In ${H_2}S{O_5}$ let oxidation state of $S = x$
We know that,
$2H + S + 5O = 0$
Oxidation state of hydrogen = $ + 1$ and oxidation state of oxygen = $ - 2$
Now on substituting the values we get,
$2( + 1) + x + 5( - 2) = 0$
$ \Rightarrow x = + 8$
This is a case of fallacy as we know that a sulphur atom cannot have an oxidation state of $ + 8$ but on observing the structure of ${H_2}S{O_5}$ we get
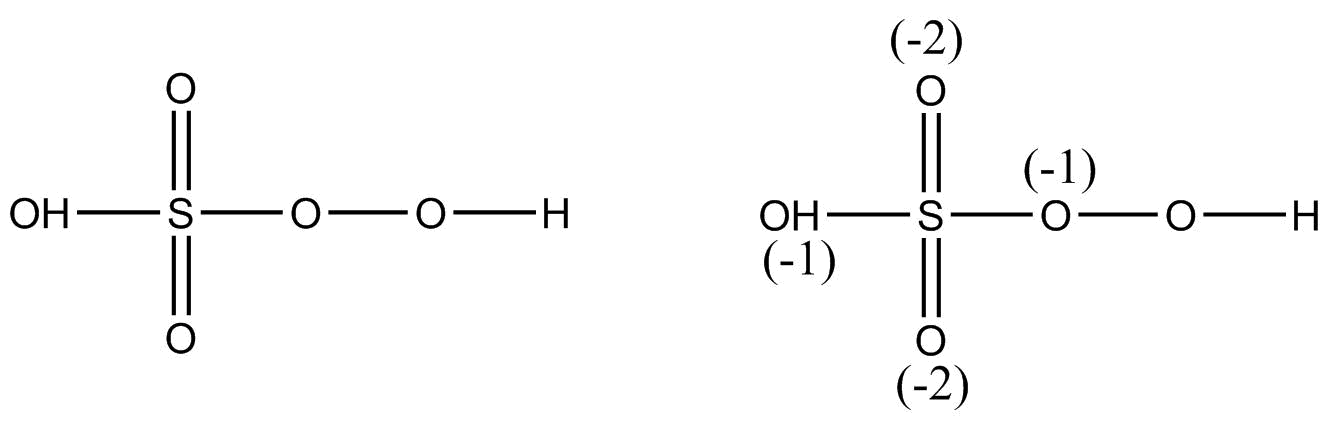
Thus, the oxidation state of sulphur in ${H_2}S{O_5}$ is $ + 6$
- In $C{r_2}O_7^{2 - }$ let oxidation state of $Cr = x$
We know that,
$2Cr + 7O = - 2$
Oxidation state of oxygen = $ - 2$
Now on substituting the values we get,
$2x + 14 = - 2$
$ \Rightarrow x = + 6$
Thus, this is not the case of fallacy and the oxidation state of Chromium in $C{r_2}O_7^{2 - }$ is $ + 6$.
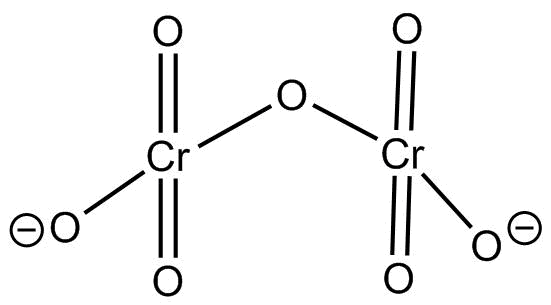
This is the structure of $C{r_2}O_7^{2 - }$
- In $N{O_3}^ - $ let oxidation state of $N = x$
We know that,
$N + 3O = - 1$
Oxidation state of oxygen = $ - 2$
Now on substituting the values we get,
$x - 6 = - 1$
$ \Rightarrow x = + 5$
Thus, this is not the case of fallacy and the oxidation state of Nitrogen in $NO_3^ - $ is $ + 5$.
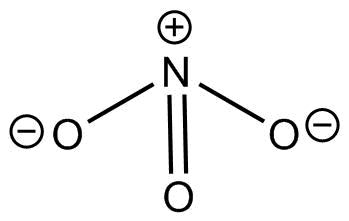
This is the structure of $NO_3^ - $
Note: ${H_2}S{O_4}$ is used as an oxidant in various processes like leaching of copper and zinc ores and is used in cyanidation of gold ores. It is known as Caro's acid. $C{r_2}O_7^{2 - }$ is a good oxidising agent which is used to oxidise alcohols. $NO_3^ - $ is used as an oxidising agent as well as to manufacture fertilisers. Nitrates are used to remove air bubbles from molten glass.
Complete step by step answer:
Oxidation state of an atom in a chemical compound is the degree of oxidation of that atom. It is indicated as the charge on an atom when all bonds of that atom are completely ionic with other elements. It is an integer value which may be positive, negative or zero. The oxidation number of a free element is zero and for hydrogen it is equal to +1 but if hydrogen forms a bond with a less electronegative element then its oxidation state is taken as -1. For oxygen oxidation state is taken as $ - 2$ but in case of peroxide it is -1.
For the given question
- In ${H_2}S{O_5}$ let oxidation state of $S = x$
We know that,
$2H + S + 5O = 0$
Oxidation state of hydrogen = $ + 1$ and oxidation state of oxygen = $ - 2$
Now on substituting the values we get,
$2( + 1) + x + 5( - 2) = 0$
$ \Rightarrow x = + 8$
This is a case of fallacy as we know that a sulphur atom cannot have an oxidation state of $ + 8$ but on observing the structure of ${H_2}S{O_5}$ we get

Thus, the oxidation state of sulphur in ${H_2}S{O_5}$ is $ + 6$
- In $C{r_2}O_7^{2 - }$ let oxidation state of $Cr = x$
We know that,
$2Cr + 7O = - 2$
Oxidation state of oxygen = $ - 2$
Now on substituting the values we get,
$2x + 14 = - 2$
$ \Rightarrow x = + 6$
Thus, this is not the case of fallacy and the oxidation state of Chromium in $C{r_2}O_7^{2 - }$ is $ + 6$.

This is the structure of $C{r_2}O_7^{2 - }$
- In $N{O_3}^ - $ let oxidation state of $N = x$
We know that,
$N + 3O = - 1$
Oxidation state of oxygen = $ - 2$
Now on substituting the values we get,
$x - 6 = - 1$
$ \Rightarrow x = + 5$
Thus, this is not the case of fallacy and the oxidation state of Nitrogen in $NO_3^ - $ is $ + 5$.

This is the structure of $NO_3^ - $
Note: ${H_2}S{O_4}$ is used as an oxidant in various processes like leaching of copper and zinc ores and is used in cyanidation of gold ores. It is known as Caro's acid. $C{r_2}O_7^{2 - }$ is a good oxidising agent which is used to oxidise alcohols. $NO_3^ - $ is used as an oxidising agent as well as to manufacture fertilisers. Nitrates are used to remove air bubbles from molten glass.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




