
${{C}_{6}}{{H}_{5}}C{{H}_{3}}\xrightarrow{Cr{{O}_{2}}C{{l}_{2}}}Z$
In the given sequence, $Z$ is:
A) Benzaldehyde
B) Toluic acid
C) Phenol acetic acid
D) Benzoic acid
Answer
527.4k+ views
Hint: The chemical formula ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$ indicates toluene (methyl group attached to benzene ring. The chemical \[Cr{{O}_{2}}C{{l}_{2}}\] is a mild oxidising agent. Hence, the above reaction shows the mild oxidation of Toluene, which means either hydrogen will be removed or oxygen will be added or both.
Complete answer:
Here, we are given a compound by the chemical formula ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$ . Now we know that the $C_6H_5-$ component shows a benzene ring. Hence, the given compound is Toluene whose structural formula is given as,
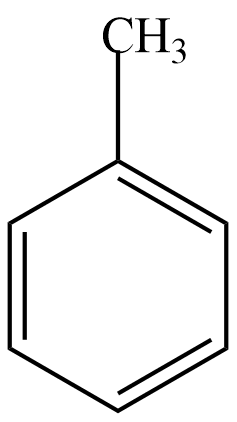
Now, the catalyst given here is \[Cr{{O}_{2}}C{{l}_{2}}\] which acts as a mild oxidising agent. Hence, we can understand the reaction will give the oxidised product of toluene.
Now, if toluene is viewed through a methyl group, it can be seen as a primary alkane. We know that oxidation of a primary alkane gives alcohol which on further oxidation gives aldehyde, which on further oxidation gives carboxylic acid.
However, if the oxidising agent is weak or in limited quantity, the oxidation stops at aldehyde. As here we are using a mild oxidising agent, hence we will get an aldehyde as a final product.
Now, the oxidation of toluene in presence of oxidising agent to produce aldehyde is shown as
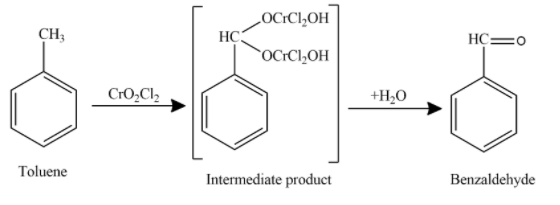
Hence, the final product obtained is Benzaldehyde. This reaction is known as Etard’s reaction
Hence, the final answer is Option $(A)$
Note: This reaction is used for the laboratory preparation of Benzaldehyde. Here, the oxidizing agent \[Cr{{O}_{2}}C{{l}_{2}}\] is a mild oxidising agent. We can also use $Cr{{O}_{3}}$ combined with ${{(C{{H}_{3}}CO)}_{2}}O$ as a mild oxidising agent. Whenever we are required to prepare aldehyde from alkane, we need a mild oxidising agent. A strong oxidising agent will carry the oxidation further and produce carboxylic acid from aldehyde.
Complete answer:
Here, we are given a compound by the chemical formula ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$ . Now we know that the $C_6H_5-$ component shows a benzene ring. Hence, the given compound is Toluene whose structural formula is given as,

Now, the catalyst given here is \[Cr{{O}_{2}}C{{l}_{2}}\] which acts as a mild oxidising agent. Hence, we can understand the reaction will give the oxidised product of toluene.
Now, if toluene is viewed through a methyl group, it can be seen as a primary alkane. We know that oxidation of a primary alkane gives alcohol which on further oxidation gives aldehyde, which on further oxidation gives carboxylic acid.
However, if the oxidising agent is weak or in limited quantity, the oxidation stops at aldehyde. As here we are using a mild oxidising agent, hence we will get an aldehyde as a final product.
Now, the oxidation of toluene in presence of oxidising agent to produce aldehyde is shown as

Hence, the final product obtained is Benzaldehyde. This reaction is known as Etard’s reaction
Hence, the final answer is Option $(A)$
Note: This reaction is used for the laboratory preparation of Benzaldehyde. Here, the oxidizing agent \[Cr{{O}_{2}}C{{l}_{2}}\] is a mild oxidising agent. We can also use $Cr{{O}_{3}}$ combined with ${{(C{{H}_{3}}CO)}_{2}}O$ as a mild oxidising agent. Whenever we are required to prepare aldehyde from alkane, we need a mild oxidising agent. A strong oxidising agent will carry the oxidation further and produce carboxylic acid from aldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




