
By using an expanded octet for the sulphur atom in thionyl chloride $(SOC{l_2})$ , you can write a Lewis structure with no formal charge.
(A) True
(B) False
Answer
559.8k+ views
Hint:To solve this question, we must first understand the whole concept of expanded octet and structure of Thionyl chloride. Then we need to use the expanded octet for the sulphur atom in thionyl chloride and then only we can have our correct answer.
Complete step-by-step answer:Expanded octet occurs when an atom is able to have more than 8 valence electrons. For example, in $S{O_3}$ , the sulfur atom forms 6 covalent bonds, hence it has 12 valence electrons. Expansion of octet is possible only from Period 3 elements onwards, due to the presence of low-lying empty d orbitals that can accommodate the extra electrons.
Let’s draw the expanded octet for the sulphur atom in thionyl chloride $(SOC{l_2})$ :
Step 1: Find the number of bonds and number of lone pairs in the thionyl chloride $(SOC{l_2})$ molecule:
Sulphur has 6 valence electrons and it will make 4 covalent bonds, a double bond with oxygen and two single bonds with chlorine, and will have a lone pair.
Step 2: So now we will draw the Lewis structure like below:
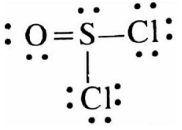
Hence it is true therefore the correct answer is (A).
Note:Thionyl chloride is an inorganic compound with the chemical formula $(SOC{l_2})$ . It is a moderately volatile colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.
Complete step-by-step answer:Expanded octet occurs when an atom is able to have more than 8 valence electrons. For example, in $S{O_3}$ , the sulfur atom forms 6 covalent bonds, hence it has 12 valence electrons. Expansion of octet is possible only from Period 3 elements onwards, due to the presence of low-lying empty d orbitals that can accommodate the extra electrons.
Let’s draw the expanded octet for the sulphur atom in thionyl chloride $(SOC{l_2})$ :
Step 1: Find the number of bonds and number of lone pairs in the thionyl chloride $(SOC{l_2})$ molecule:
Sulphur has 6 valence electrons and it will make 4 covalent bonds, a double bond with oxygen and two single bonds with chlorine, and will have a lone pair.
Step 2: So now we will draw the Lewis structure like below:

Hence it is true therefore the correct answer is (A).
Note:Thionyl chloride is an inorganic compound with the chemical formula $(SOC{l_2})$ . It is a moderately volatile colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




