
Buff colored precipitate is obtained when $FeC{{l}_{3}}$ is treated with:
(a)-
(b)-
(c)-
(d)-




Answer
578.1k+ views
Hint: When any compound on treatment with ferric chloride gives a buff-colored solution then the compound is having the phenol group. This is known as the ferric chloride test.
Complete answer:
If a compound can buff-colored precipitate with ferric chloride, then the compound gives a positive result for the ferric chloride test. This test is used to detect the presence of the phenol group in the compound i.e., if the compound contains phenol group then it will give positive results for the ferric chloride test.
Phenol group means when an alcohol group is directly attached to one of the carbon atoms of the benzene ring.
This test is performed by mixing the sample compound with either water or ethanol and then adding a little amount of dilute ferric chloride solution. On adding the ferric chloride, if the presence of red, blue, green, or purple colored precipitate is formed when the compound contains a phenol group. If the sample is insoluble in water and ethanol then the compound is mixed in dichloromethane with a small amount of pyridine.
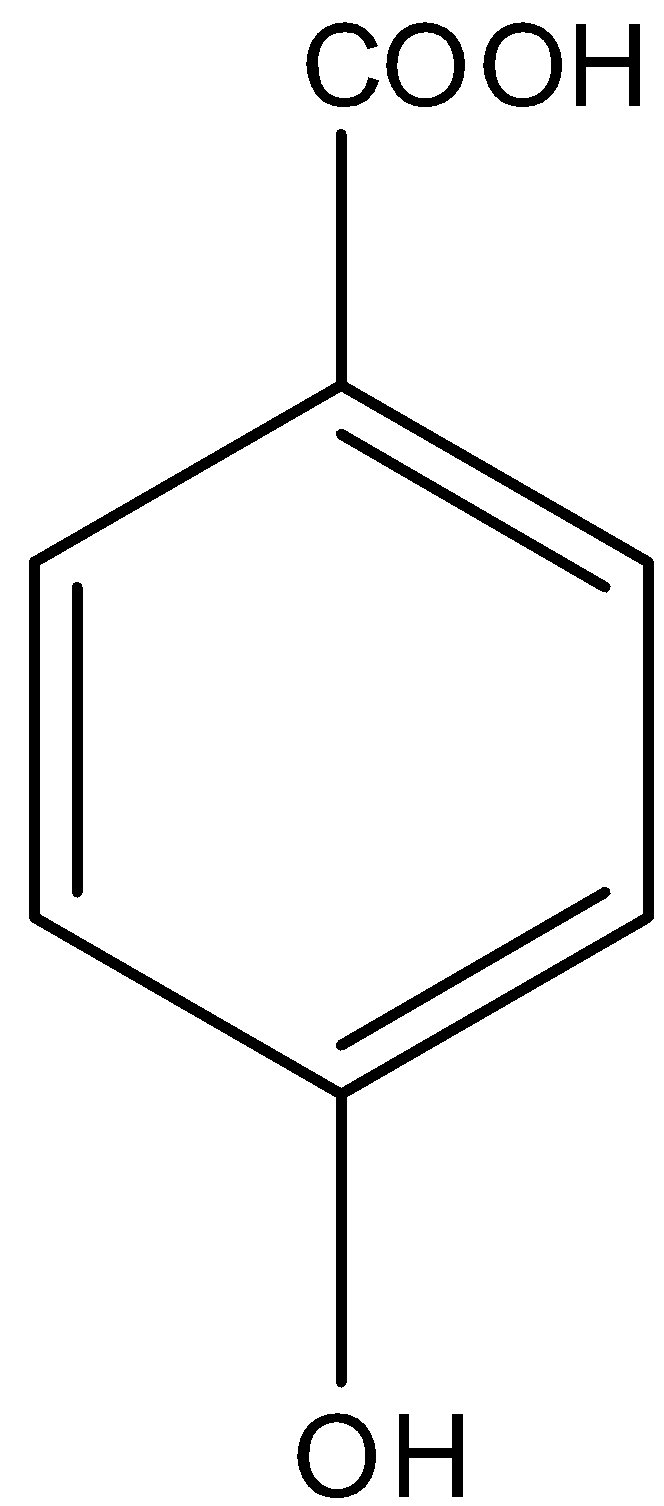
So in
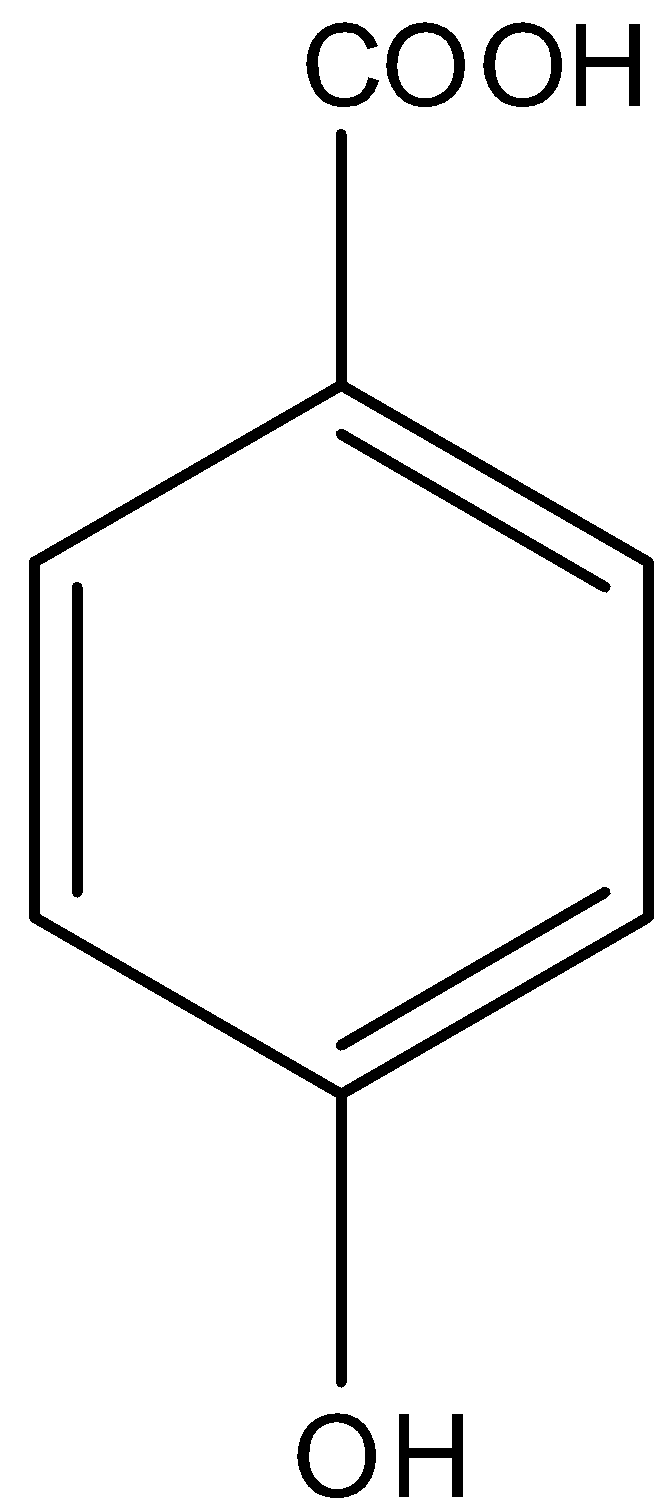
There is a phenol group, it will give the ferric chloride test.
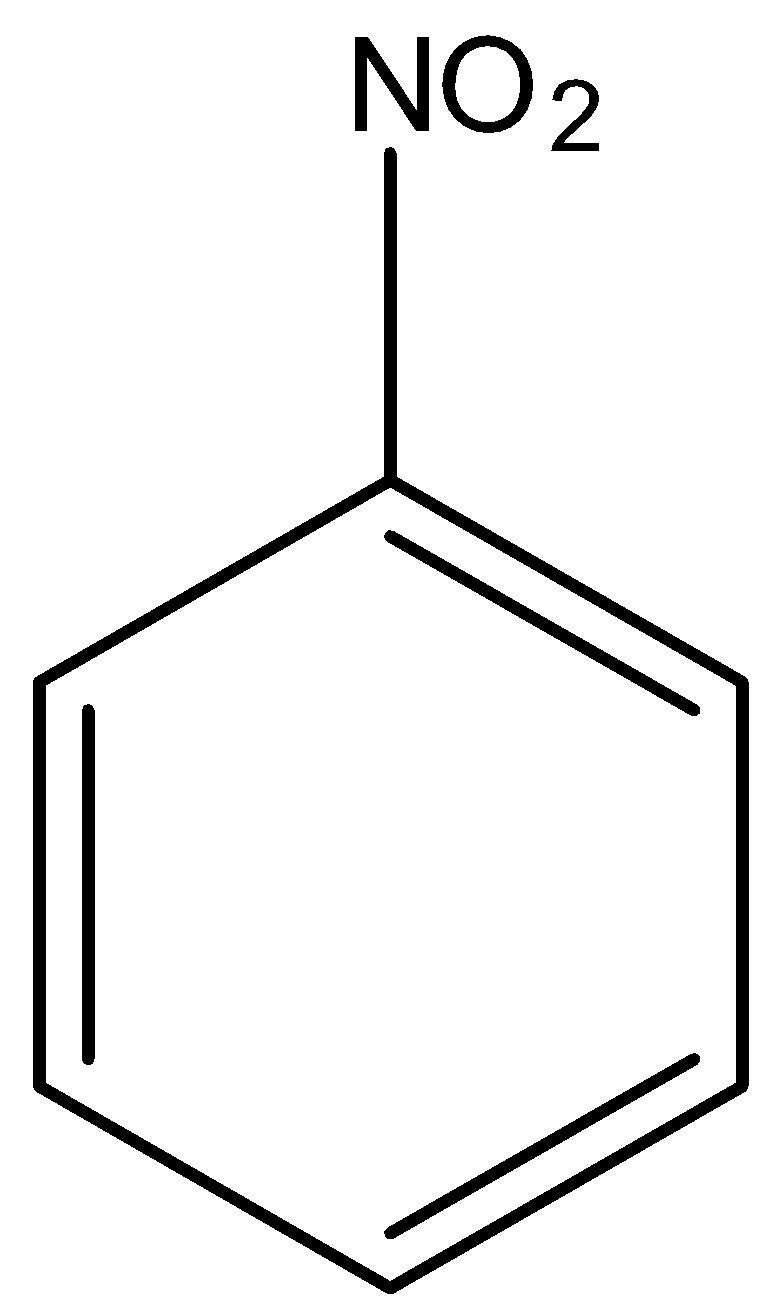
In
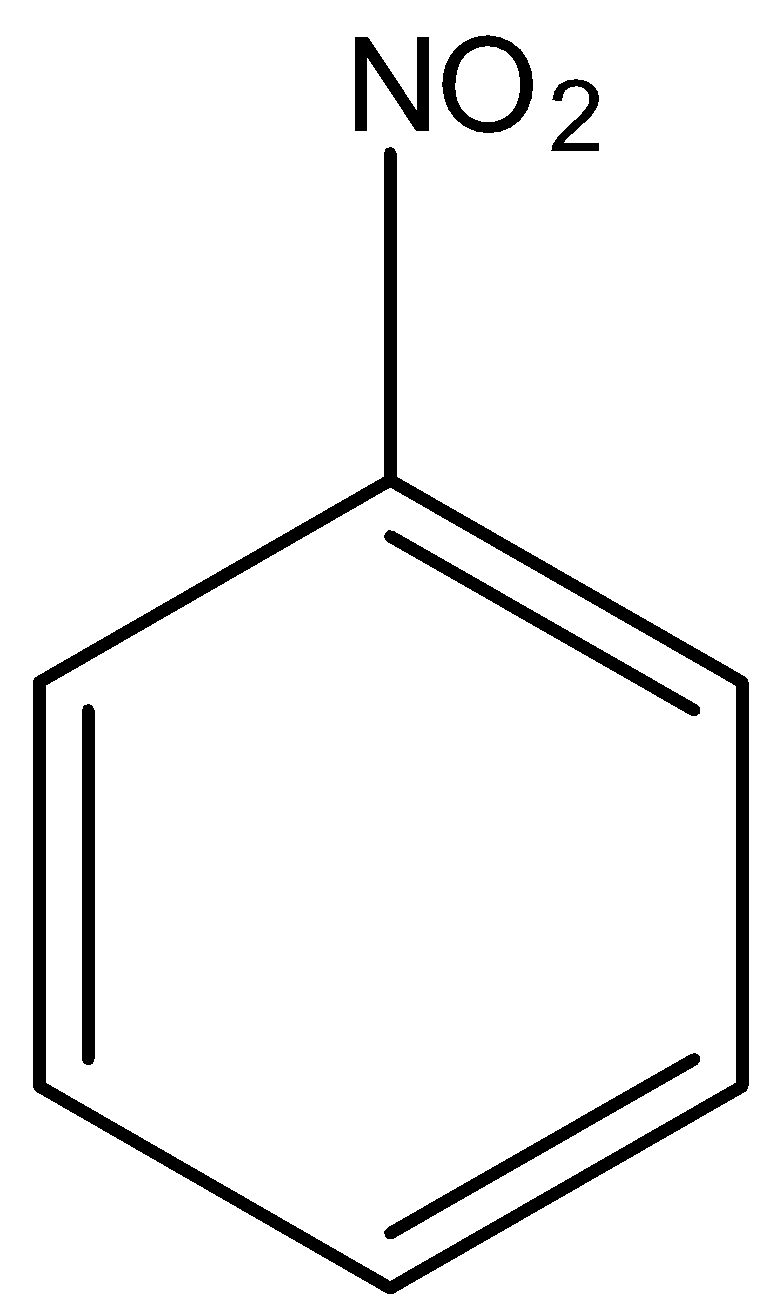
There is no phenol group, it will not give the ferric chloride test.
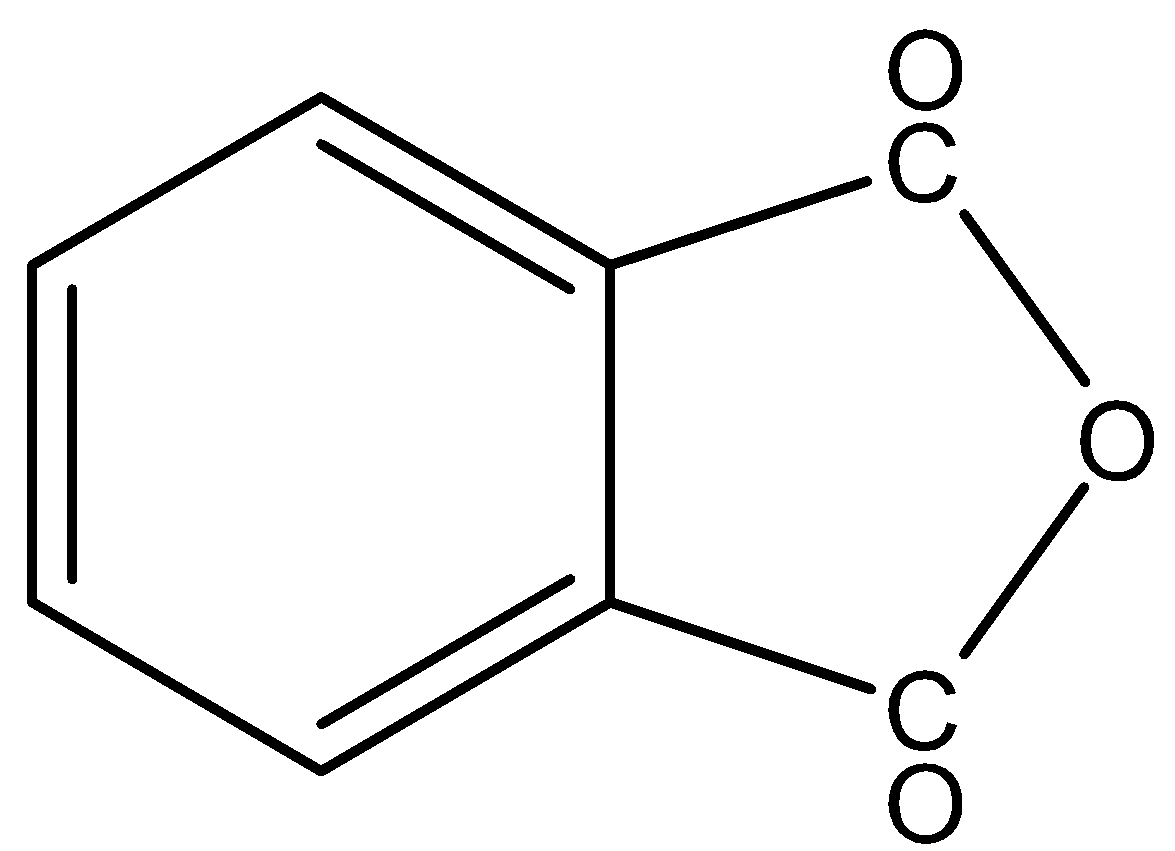
In,
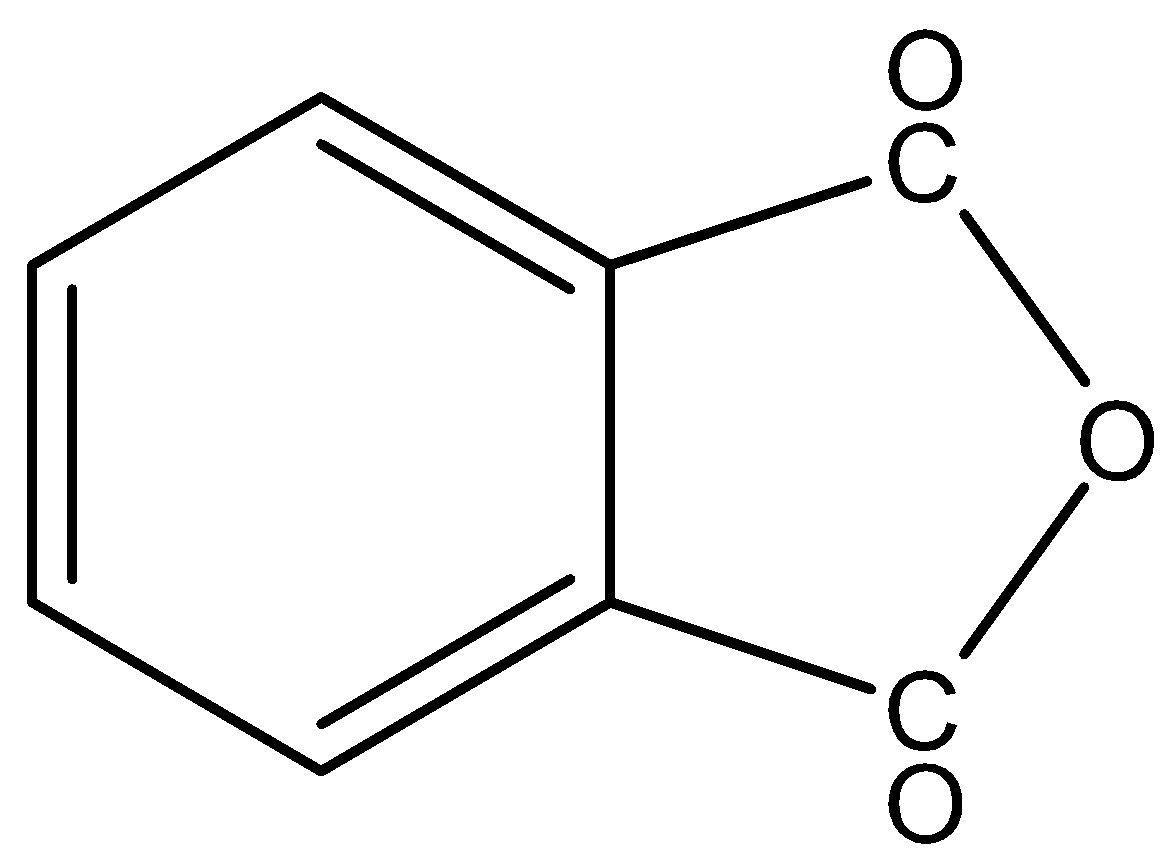
There is no phenol group, it will not give the ferric chloride test.
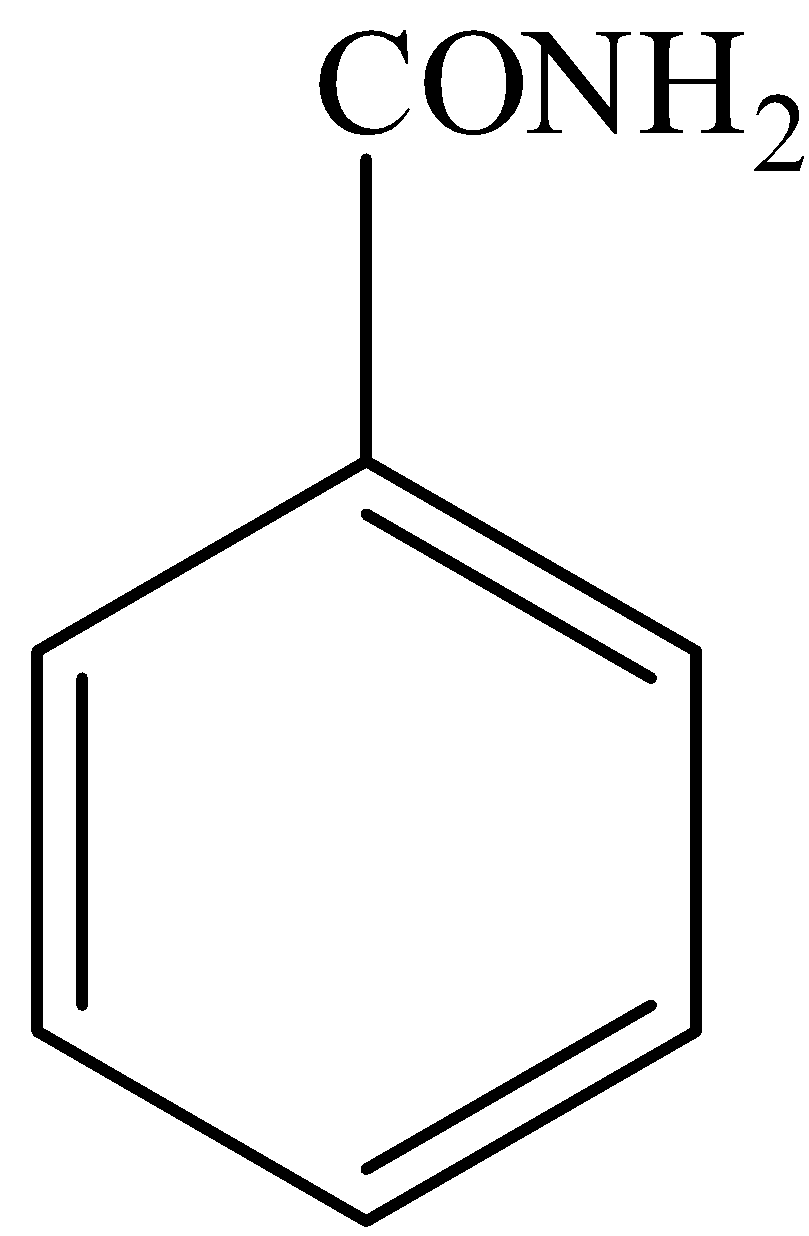
In,
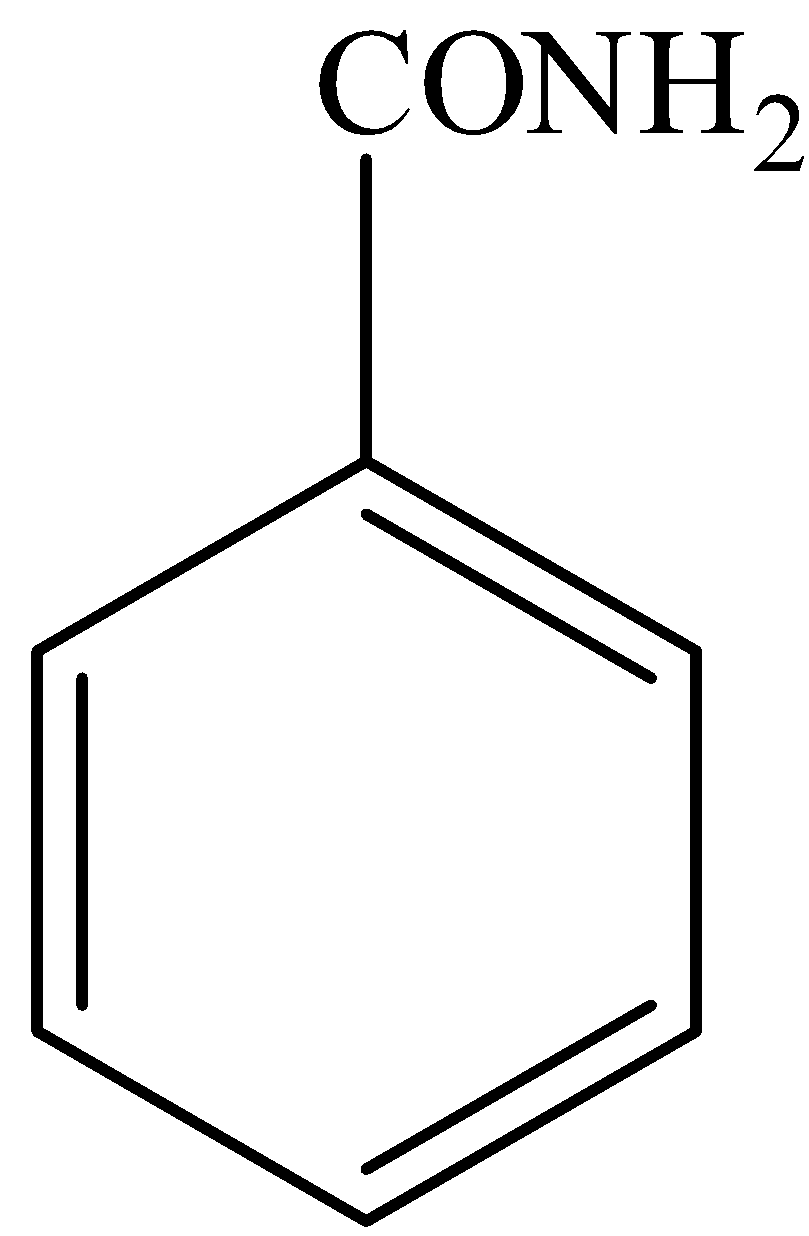
There is no phenol group, it will not give the ferric chloride test.
Therefore, the correct answer is option (a).
Note:
It depends on the nature of the phenol that whether the precipitate formed will be of red, blue, green, or purple color. The color is due to the formation of complex ferric ions with phenols.
Complete answer:
If a compound can buff-colored precipitate with ferric chloride, then the compound gives a positive result for the ferric chloride test. This test is used to detect the presence of the phenol group in the compound i.e., if the compound contains phenol group then it will give positive results for the ferric chloride test.
Phenol group means when an alcohol group is directly attached to one of the carbon atoms of the benzene ring.
This test is performed by mixing the sample compound with either water or ethanol and then adding a little amount of dilute ferric chloride solution. On adding the ferric chloride, if the presence of red, blue, green, or purple colored precipitate is formed when the compound contains a phenol group. If the sample is insoluble in water and ethanol then the compound is mixed in dichloromethane with a small amount of pyridine.
So in

There is a phenol group, it will give the ferric chloride test.
In

There is no phenol group, it will not give the ferric chloride test.
In,

There is no phenol group, it will not give the ferric chloride test.
In,

There is no phenol group, it will not give the ferric chloride test.
Therefore, the correct answer is option (a).
Note:
It depends on the nature of the phenol that whether the precipitate formed will be of red, blue, green, or purple color. The color is due to the formation of complex ferric ions with phenols.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




