
Bring out the following conversion: Ethyl cyanide to 1-phenyl propanone.
Answer
573.3k+ views
Hint:A nitrile group which is commonly known as cyanide group is converted into a carbonyl group (ketone) takes place with the help of Grignard reagents which is represented by R-MgX- where R could be an alkyl or aryl group which acts as a strong base and a strong nucleophile. X represents the halogen atom.
Complete answer: Grignard reagents are an organo- metallic halogen compounds, it performs acid base reactions and nucleophilic addition reactions. Ethyl cyanides gives an additional product having one or more carbon atoms with Grignard reagent, which is later on hydrolysis gives 1- phenyl propanone.
\[R-CN\,+\,{{R}_{1}}^{-}Mg{{X}^{-}}\,\,\to \,\,R-C{{R}_{2}}={{N}^{-}}MgBr\]
R1 and R2 represent two different alkyl groups. This reaction is completed in two steps. In the first step the phenyl group of Grignard reagent acts as a nucleophile. Due to the driving force of the reaction nucleophile attack the cyanide group, multiple bonds of cyanide group migrate toward the more electronegative atom, so that nitrogen atom of cyanide group gets extra negative charge and carbon atom gets positive charge. Phenyl group gets attached with a positively charged carbon atom and negative part of the Grignard reagent attached with a nitrogen atom. In second this intermediate is followed by hydrolysis and produces 1-phenyl propanone.
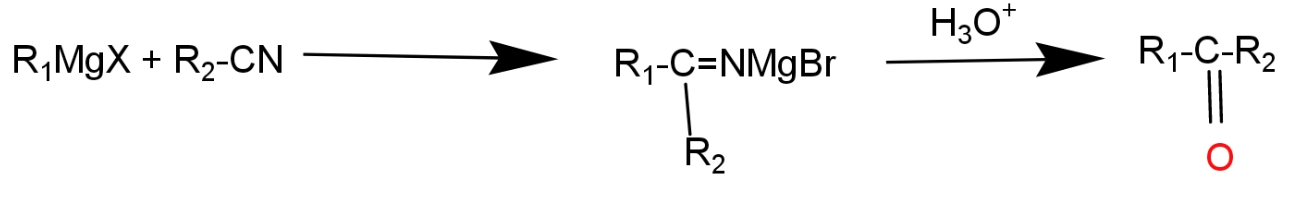
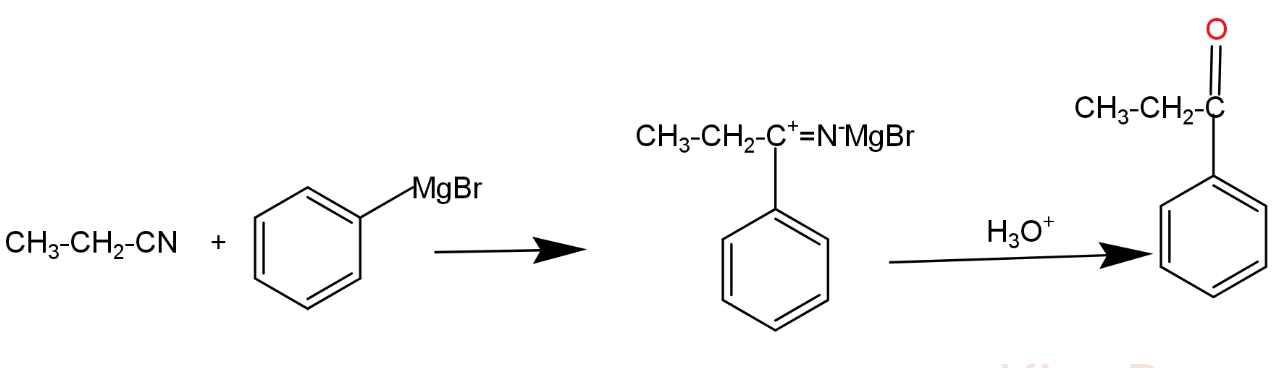
Note: Grignard reagents are highly reactive compounds and it can react with any source of proton to give hydrocarbons because acid base reactions are much faster than addition reactions. So it is essential to avoid even a small amount of moisture from a Grignard reagent.
Complete answer: Grignard reagents are an organo- metallic halogen compounds, it performs acid base reactions and nucleophilic addition reactions. Ethyl cyanides gives an additional product having one or more carbon atoms with Grignard reagent, which is later on hydrolysis gives 1- phenyl propanone.
\[R-CN\,+\,{{R}_{1}}^{-}Mg{{X}^{-}}\,\,\to \,\,R-C{{R}_{2}}={{N}^{-}}MgBr\]
R1 and R2 represent two different alkyl groups. This reaction is completed in two steps. In the first step the phenyl group of Grignard reagent acts as a nucleophile. Due to the driving force of the reaction nucleophile attack the cyanide group, multiple bonds of cyanide group migrate toward the more electronegative atom, so that nitrogen atom of cyanide group gets extra negative charge and carbon atom gets positive charge. Phenyl group gets attached with a positively charged carbon atom and negative part of the Grignard reagent attached with a nitrogen atom. In second this intermediate is followed by hydrolysis and produces 1-phenyl propanone.


Note: Grignard reagents are highly reactive compounds and it can react with any source of proton to give hydrocarbons because acid base reactions are much faster than addition reactions. So it is essential to avoid even a small amount of moisture from a Grignard reagent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




