
How will you bring about the following conversations?
(i) Propanone to propane
(ii) Benzoyl chloride to benzaldehyde
(iii) Ethanal to but-2-enal.
Answer
591.9k+ views
Hint: Following changes are occurring:
(i) The carbonyl group is reduced to methylene group.
(ii) Acid chloride group is converted to an aldehyde group.
(iii) Two molecules of aldehyde condense in this conversion.
Complete step by step answer:
The conversion of propanone to propane can be carried out with either Clemmensen reduction or Wolff-Kishner reduction.
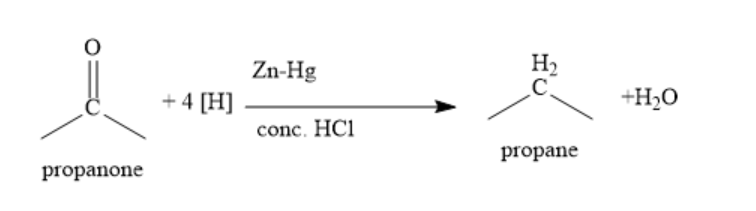
In Clemmensen reduction, propanone is treated with zinc amalgam and concentrated hydrochloric acid. Carbonyl group is reduced to methylene group.
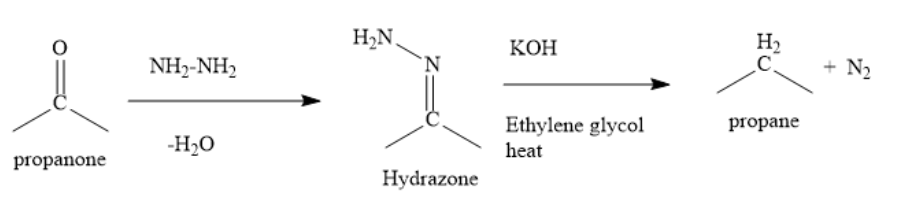
In Wolff-Kishner reduction, propanone is treated with hydrazine, followed by potassium hydroxide in a high boiling solvent such as ethylene glycol. Carbonyl group is reduced to methylene group.
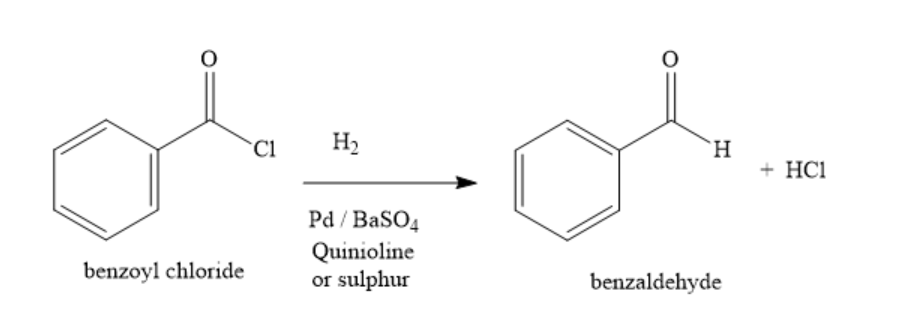
The conversion of benzoyl chloride to benzaldehyde is carried out by Rosenmund reduction. Benzoyl chloride is treated with hydrogen and palladium- barium sulphate catalyst partially poisoned with quinoline or sulphur. Acid chloride group is converted into aldehyde group.
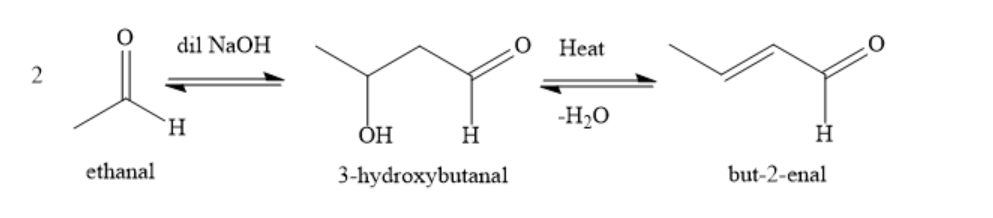
The conversion of ethanal to but-2-enal is carried out by aldol condensation reaction. Ethanol is acetaldehyde and contains an alpha hydrogen atom. In presence of dilute alkali, two molecules of ethanal condense to form 3-hydroxybutanal.
Upon heating, this 3-hydroxybutanal loses a water molecule to form but-2-enal.
Note:
(i) Do not use the reagents that reduce carbonyl groups to alcohols.
(ii) Do not use the reagents that convert an acid chloride into an ester or a carboxylic acid.
(iii) Do not forget to add the step for water elimination.
(ii) Acid chloride group is converted to an aldehyde group.
(iii) Two molecules of aldehyde condense in this conversion.
Complete step by step answer:
The conversion of propanone to propane can be carried out with either Clemmensen reduction or Wolff-Kishner reduction.
In Clemmensen reduction, propanone is treated with zinc amalgam and concentrated hydrochloric acid. Carbonyl group is reduced to methylene group.

In Wolff-Kishner reduction, propanone is treated with hydrazine, followed by potassium hydroxide in a high boiling solvent such as ethylene glycol. Carbonyl group is reduced to methylene group.

The conversion of benzoyl chloride to benzaldehyde is carried out by Rosenmund reduction. Benzoyl chloride is treated with hydrogen and palladium- barium sulphate catalyst partially poisoned with quinoline or sulphur. Acid chloride group is converted into aldehyde group.

The conversion of ethanal to but-2-enal is carried out by aldol condensation reaction. Ethanol is acetaldehyde and contains an alpha hydrogen atom. In presence of dilute alkali, two molecules of ethanal condense to form 3-hydroxybutanal.
Upon heating, this 3-hydroxybutanal loses a water molecule to form but-2-enal.

Note:
(i) Do not use the reagents that reduce carbonyl groups to alcohols.
(ii) Do not use the reagents that convert an acid chloride into an ester or a carboxylic acid.
(iii) Do not forget to add the step for water elimination.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




