
Both phosphinic acid and phosphonic acids have
A. One $P=O$ bond
B. Two $P-H$ bond
C. Two $P-OH$ bond
D. One $P-O-P$ bond
Answer
567k+ views
Hint: Phosphinic or phosphonic acids both are phosphorous oxyacid. Phosphinic acid also known by the name hypophosphorous acid is generally colorless low melting point while phosphonic acid can be known as the phosphonates these are generally organophosphorus compounds.
Complete answer: Phosphinic acid is represented by the molecular formula ${{H}_{3}}P{{O}_{2}}$ which is used as a reducing agent. It is soluble in water, dioxane and alcohol. It generally represents in the form of $HOP(O){{H}_{2}}$ which defines its monoprotic character or we can say acidic character. At equilibrium $HOP(O){{H}_{2}}$ exists with the minor tautomer $HP{{(OH)}_{2}}$. The structure of phosphonic acid can be shown as:

Phosphonic acids are also known by the name phosphonates and these are kept in the category of organophosphorus compounds. Phosphonic acids are represented by the molecular formula containing $R-P{{O}_{3}}{{H}_{2}}$ group where R may be an alkyl or aryl group. Phosphonic acid consists of a single pentavalent phosphorus covalently bound via single bonds to single hydrogen and two hydroxyl groups via a double bond to an oxygen. Structure of phosphonic acid can be shown as:
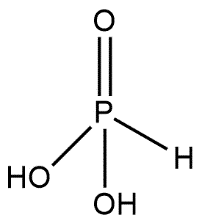
In both of the structures we can consider that one $P=O$ bond is common.
Hence we can say that option A is the correct answer.
Note: The main use of Phosphinic acid is its industrial use for electroless nickel plating but it can be used as a salt. It also reduces chromium(III) oxide to chromium(II) oxide. Phosphonic acid is a conjugate acid of a phosphonate and a tautomer of a phosphorous acid.
Complete answer: Phosphinic acid is represented by the molecular formula ${{H}_{3}}P{{O}_{2}}$ which is used as a reducing agent. It is soluble in water, dioxane and alcohol. It generally represents in the form of $HOP(O){{H}_{2}}$ which defines its monoprotic character or we can say acidic character. At equilibrium $HOP(O){{H}_{2}}$ exists with the minor tautomer $HP{{(OH)}_{2}}$. The structure of phosphonic acid can be shown as:

Phosphonic acids are also known by the name phosphonates and these are kept in the category of organophosphorus compounds. Phosphonic acids are represented by the molecular formula containing $R-P{{O}_{3}}{{H}_{2}}$ group where R may be an alkyl or aryl group. Phosphonic acid consists of a single pentavalent phosphorus covalently bound via single bonds to single hydrogen and two hydroxyl groups via a double bond to an oxygen. Structure of phosphonic acid can be shown as:

In both of the structures we can consider that one $P=O$ bond is common.
Hence we can say that option A is the correct answer.
Note: The main use of Phosphinic acid is its industrial use for electroless nickel plating but it can be used as a salt. It also reduces chromium(III) oxide to chromium(II) oxide. Phosphonic acid is a conjugate acid of a phosphonate and a tautomer of a phosphorous acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




