
Boiling point of nitromethane is:
(A) $370K$
(B) $374K$
(C) $378K$
(D) $379K$
Answer
563.1k+ views
Hint: We know that nitromethane is a nitro alkane which is obtained by treating alkyl halides with silver nitrate in an alcoholic solution. By the hydrolysis of $\alpha - $ nitro alkene we can easily produce nitromethane. Nitro methane can also be produced by reacting methane with concentrated nitric acid at high temperature.
Complete step-by-step answer:Nitromethane is a colorless, slightly viscous and a highly polar compound. We know that polar molecules are those compounds in which a partial negative charge and a partial positive charge is acquired by the atoms of the molecule. due to the difference of electronegativity between the atoms or due to the lone pairs present on the central metal atom.
We know that nitrogen possesses lone pairs due to which the polar nature arises and also the electronegativity of oxygen is more than that of nitrogen.
Structure of nitromethane:
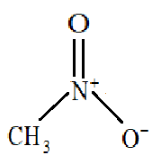
So, nitro methane will be highly polar and we know that water is also a highly polar compound therefore the boiling point of nitromethane will be very similar to that of water.
The boiling point of water is $100^\circ C$ or $373K$ and among the given options the closest boiling point to that of water is $374K$
Therefore, the correct answer is Option (B).
Note: High boiling point means that intermolecular hydrogen bonding will be present which will be very strong. Due to that strong force of attraction, it will be very difficult for the liquid to escape into vapor phase i.e., more energy will be needed to convert the liquid into vapor. Nitroalkanes are usually soluble in water.
Complete step-by-step answer:Nitromethane is a colorless, slightly viscous and a highly polar compound. We know that polar molecules are those compounds in which a partial negative charge and a partial positive charge is acquired by the atoms of the molecule. due to the difference of electronegativity between the atoms or due to the lone pairs present on the central metal atom.
We know that nitrogen possesses lone pairs due to which the polar nature arises and also the electronegativity of oxygen is more than that of nitrogen.
Structure of nitromethane:

So, nitro methane will be highly polar and we know that water is also a highly polar compound therefore the boiling point of nitromethane will be very similar to that of water.
The boiling point of water is $100^\circ C$ or $373K$ and among the given options the closest boiling point to that of water is $374K$
Therefore, the correct answer is Option (B).
Note: High boiling point means that intermolecular hydrogen bonding will be present which will be very strong. Due to that strong force of attraction, it will be very difficult for the liquid to escape into vapor phase i.e., more energy will be needed to convert the liquid into vapor. Nitroalkanes are usually soluble in water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




