
How many benzylic hydrogens are present in the hydrocarbon shown?
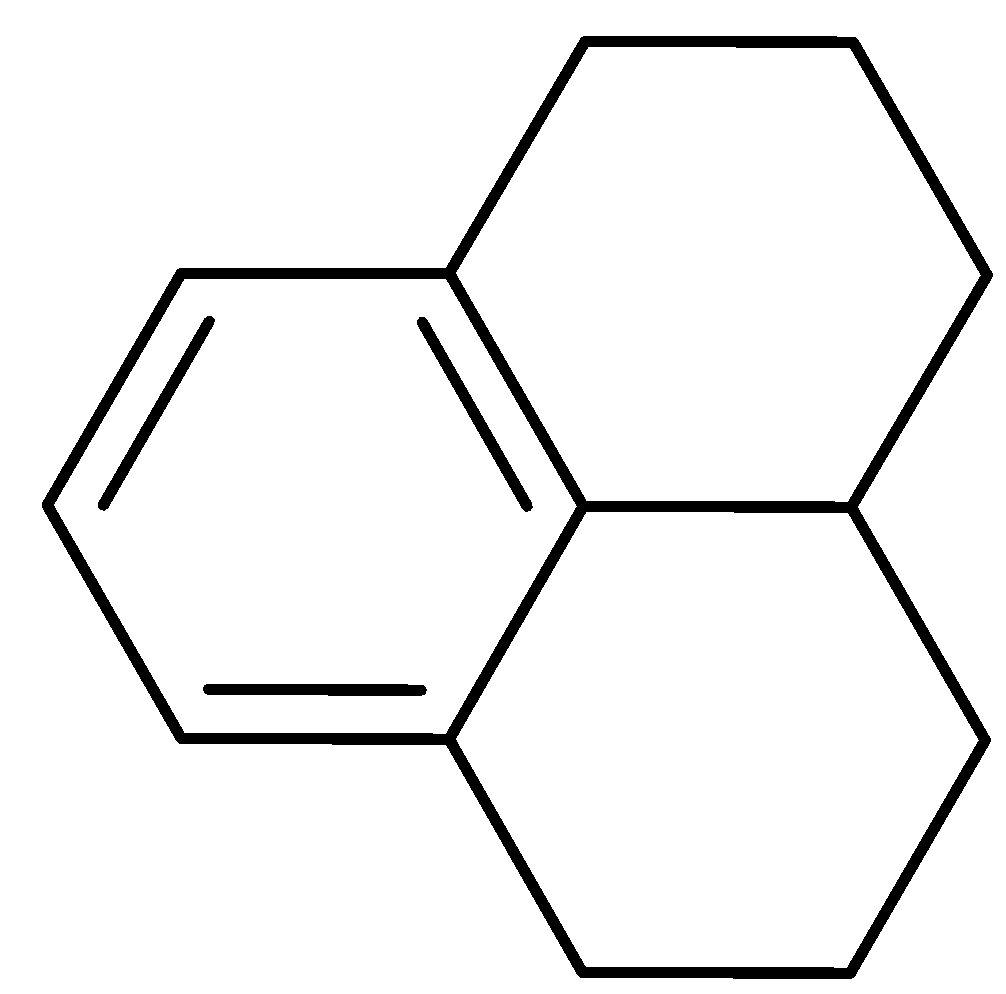
A. \[3\]
B. \[4\]
C. $5$
D. $6$
E. $8$

Answer
592.5k+ views
Hint: In organic chemistry, Benzylic hydrogens refer to the hydrogen atoms that are attached to the carbon atom just next to or in other words the carbon atom adjacent to the benzene group. For example: In ethyl benzene carbon next to benzene has two hydrogen atoms only.
Complete step by step answer:
As we know what Benzylic hydrogens are let us try to count the benzylic hydrogens present in the given molecule, the carbon atoms next to benzene are three, now as we have identified the carbon atoms let’s count the hydrogen atoms directly attached to those carbon, the total comes out to be \[2 + 1 + 2 = {\text{ }}5\] benzylic hydrogens. The carbons next to benzene atoms are encircled and the benzylic hydrogens are shown in the figure below:
So, the correct answer is Option C.
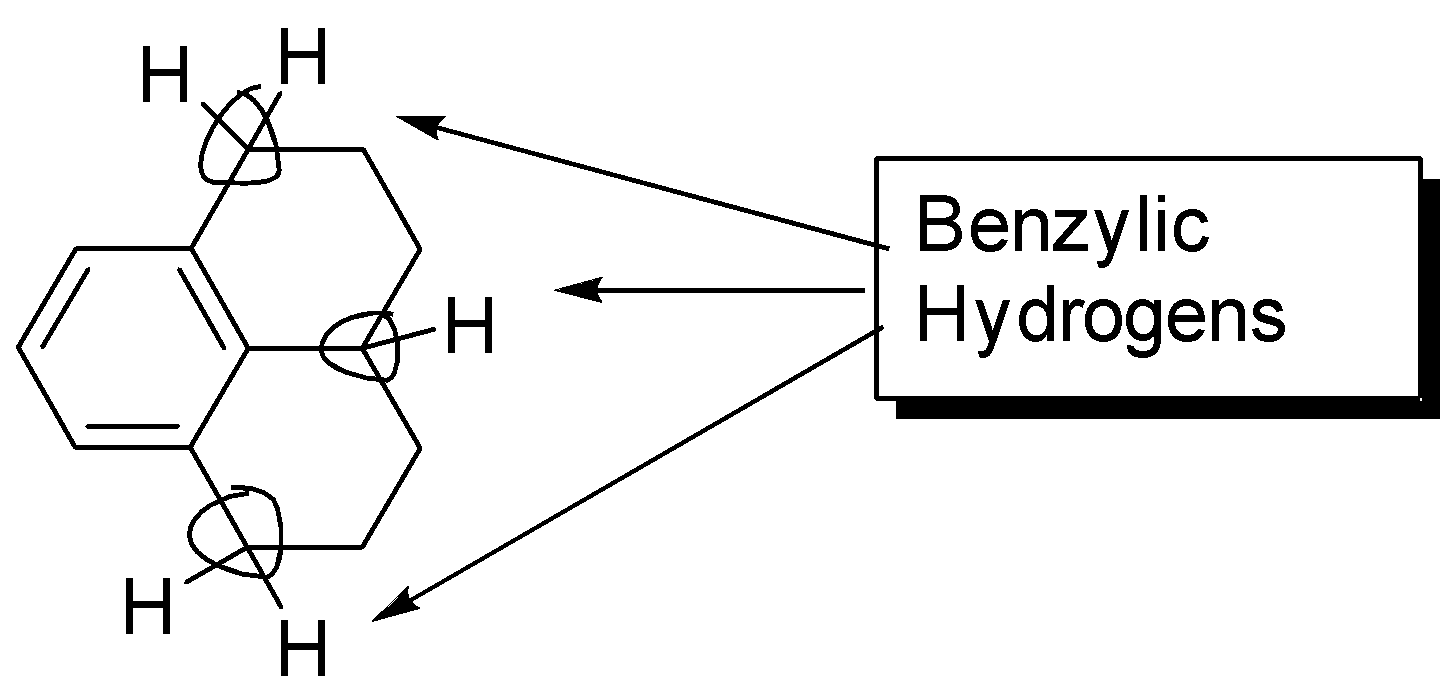
Additional information:
The benzylic hydrogens are far more very important when it comes to organic chemistry or reactions, as these being one of the most reactive sites when a functional group is present at benzylic sites as Functional groups in a benzylic position are generally more reactive than that of the isolated functional group as at the Benzylic C-H addition of halogen is easily possible via free radical path as the benzylic radical is stabilized because of the resonance (a stabilizing phenomenon occurring when three or more than three adjacent pi orbitals are present).
Note:
A student has to be careful at the time of counting the benzylic hydrogens, first of all a student should identify the adjacent carbons to the benzene ring in the case of complex structures, followed by the counting of hydrogen atoms.
Complete step by step answer:
As we know what Benzylic hydrogens are let us try to count the benzylic hydrogens present in the given molecule, the carbon atoms next to benzene are three, now as we have identified the carbon atoms let’s count the hydrogen atoms directly attached to those carbon, the total comes out to be \[2 + 1 + 2 = {\text{ }}5\] benzylic hydrogens. The carbons next to benzene atoms are encircled and the benzylic hydrogens are shown in the figure below:
So, the correct answer is Option C.

Additional information:
The benzylic hydrogens are far more very important when it comes to organic chemistry or reactions, as these being one of the most reactive sites when a functional group is present at benzylic sites as Functional groups in a benzylic position are generally more reactive than that of the isolated functional group as at the Benzylic C-H addition of halogen is easily possible via free radical path as the benzylic radical is stabilized because of the resonance (a stabilizing phenomenon occurring when three or more than three adjacent pi orbitals are present).
Note:
A student has to be careful at the time of counting the benzylic hydrogens, first of all a student should identify the adjacent carbons to the benzene ring in the case of complex structures, followed by the counting of hydrogen atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




