
Benzoyl chloride is prepared from benzoic acid by:
A. $C{l_2}$ and $h\nu $
B. $S{O_2}C{l_2}$
C. $SOC{l_2}$
D. $C{l_2}$ and ${H_2}O$
Answer
558.9k+ views
Hint: Benzoyl chloride has molecular formula ${C_6}{H_5}COCl$ with structure
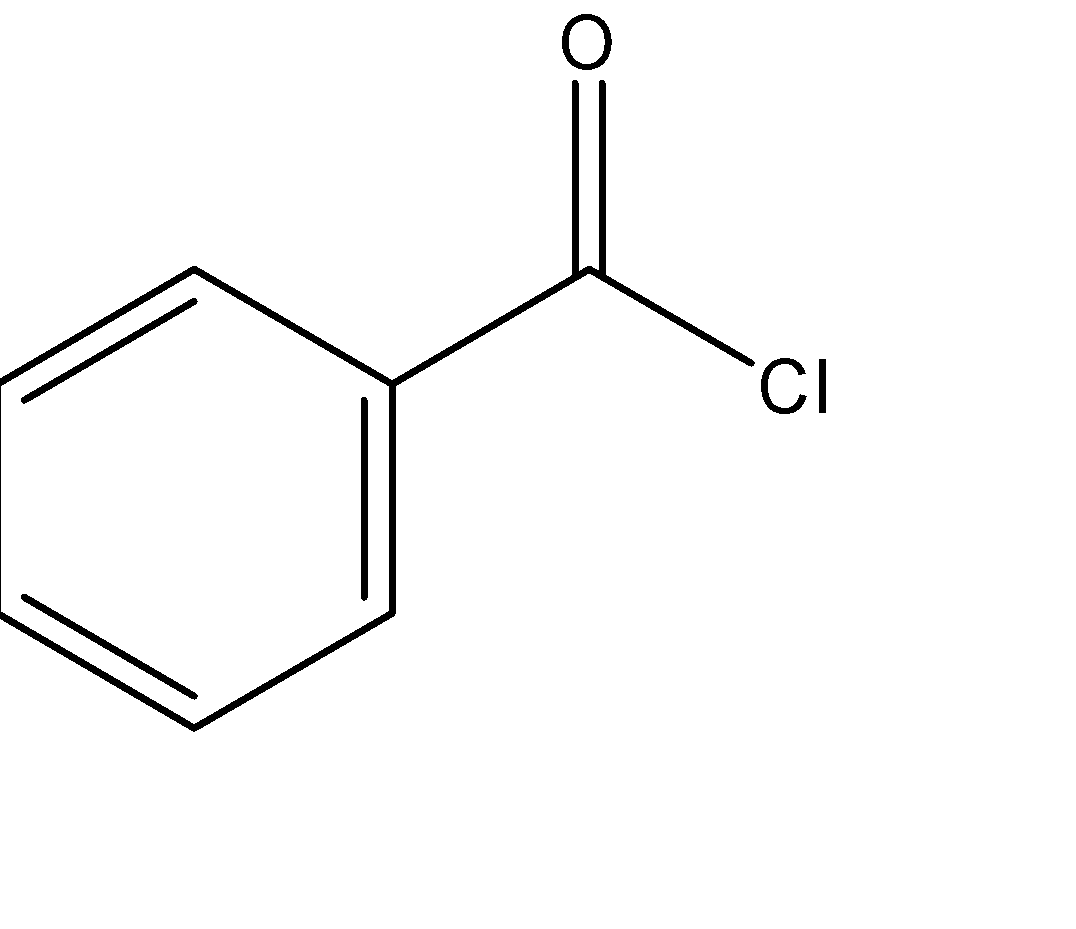
Benzoic acid is ${C_6}{H_5}COOH$
Benzoyl chloride can be prepared from benzoic acid. Here we can see that the OH group in benzoic acid is replaced by Cl in benzoyl chloride. Therefore a Chlorine compound is added.
Complete step by step answer:
Option A is a method of addition of chlorine atom to a compound. But it is not applicable here as there is no alkane molecule here. If it is present then, $C{l_2}$ and $h\nu $ can act as a chlorinating agent. If there is $C{H_3} - C{H_2} - $here, $C{l_2}$ and $h\nu $ can add a chlorine atom.
Here, benzoyl chloride can be obtained by treated benzoic acid with $SOC{l_2}$. Here, $S{O_2}^ + HCl$ is removed. $SOC{l_2}$ is also called thionyl chloride. Thionyl chloride readily replaces OH to Cl.
Benzoic acid does not react with $C{l_2}$ and ${H_2}O$
$PhCOOH\xrightarrow{{SOC{l_2}}}PhCOCl + S{O_2} + HCl$
So, the correct answer is Option C.
Note: There are many other methods of preparing benzoyl chloride. The main use of thionyl chloride is as a chlorinating agent.
Benzoyl chloride can also be prepared from benzoic acid by treating it with $PC{l_5}$.
Similarly, if the benzoic acid is treated with benzyl trichloride, benzoyl chloride can be obtained.
Thus, benzoic acid can be used to prepare benzoyl chloride by treating it with phosphorus pentachloride, thionyl chloride or benzyl trichloride.

Benzoic acid is ${C_6}{H_5}COOH$
Benzoyl chloride can be prepared from benzoic acid. Here we can see that the OH group in benzoic acid is replaced by Cl in benzoyl chloride. Therefore a Chlorine compound is added.
Complete step by step answer:
Option A is a method of addition of chlorine atom to a compound. But it is not applicable here as there is no alkane molecule here. If it is present then, $C{l_2}$ and $h\nu $ can act as a chlorinating agent. If there is $C{H_3} - C{H_2} - $here, $C{l_2}$ and $h\nu $ can add a chlorine atom.
Here, benzoyl chloride can be obtained by treated benzoic acid with $SOC{l_2}$. Here, $S{O_2}^ + HCl$ is removed. $SOC{l_2}$ is also called thionyl chloride. Thionyl chloride readily replaces OH to Cl.
Benzoic acid does not react with $C{l_2}$ and ${H_2}O$
$PhCOOH\xrightarrow{{SOC{l_2}}}PhCOCl + S{O_2} + HCl$
So, the correct answer is Option C.
Note: There are many other methods of preparing benzoyl chloride. The main use of thionyl chloride is as a chlorinating agent.
Benzoyl chloride can also be prepared from benzoic acid by treating it with $PC{l_5}$.
Similarly, if the benzoic acid is treated with benzyl trichloride, benzoyl chloride can be obtained.
Thus, benzoic acid can be used to prepare benzoyl chloride by treating it with phosphorus pentachloride, thionyl chloride or benzyl trichloride.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




