
When benzene-1,3-dicarbaldehyde reacts with 50% of $NaOH$, the possible products formed is/are:
A.
B.
C.
D.




Answer
573.3k+ views
Hint:It is a Cannizzaro reaction, when a self oxidation reduction reaction in which aldehydes that do not have any $\alpha $-hydrogen atom undergo disproportionation reaction (i.e., self-redox reaction) in the presence of 50% aqueous or ethanolic solution of alkali in which one of the molecules being reduced to alcohol and other being oxidised to the salt of the corresponding acid.
Complete answer:
We are given benzene-1,3-dialdehyde which has a structure like this.

At both $\alpha $-position of the compound that is 1 and 3, we don’t have a hydrogen atom. So the condition for Cannizzaro reaction is true and we have a 50% of $NaOH$. Hence the Cannizzaro reaction will occur here. Also since there are two carbonyl groups it undergoes intramolecular Cannizzaro reaction.
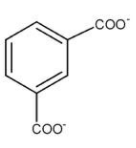
As we know that it is a disproportionation reaction, both oxidation and reduction happen in the molecule. When the carbonyl compound is attached to 1st and 3rd position of the benzene it gets oxidised to form $ - CO{O^ - }$. So we understood that at position 1 and 3 at some point of time we will have $ - CO{O^ - }$ attached, hence option (a) is a possible product.
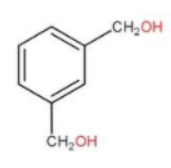
Also when benzene-1,3-dialdehyde gets reduced to form alcohols at position 1 and 3 we get $ - C{H_2}OH$. Hence option (b) is also a possible product.
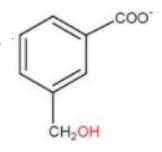
And on the intermediate of this, it may also occur at some point of time one $ - CO{O^ - }$ and one $ - C{H_2}OH$ is formed. Therefore, option (c) is also a possible product.
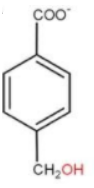
Now, coming to option(d) we can see that the position of $ - CO{O^ - }$ and $ - C{H_2}OH$are at 1 and 4 respectively, which is never going to happen as the carbonyl group are present and 1 and 3 position of the compound. Hence this product never forms.
Hence, options A,B and C are correct.
Note:
In the presence of $\alpha $-hydrogen the reaction occurred is known as aldol condensation. In this reaction, two molecules of aldehydes or ketones having $\alpha $-hydrogen condense together in presence of a base like $NaOH$ to form $\beta $-hydroxy aldehyde or $\beta $-hydroxy ketone respectively which are collectively known as aldol.
Complete answer:
We are given benzene-1,3-dialdehyde which has a structure like this.

At both $\alpha $-position of the compound that is 1 and 3, we don’t have a hydrogen atom. So the condition for Cannizzaro reaction is true and we have a 50% of $NaOH$. Hence the Cannizzaro reaction will occur here. Also since there are two carbonyl groups it undergoes intramolecular Cannizzaro reaction.
As we know that it is a disproportionation reaction, both oxidation and reduction happen in the molecule. When the carbonyl compound is attached to 1st and 3rd position of the benzene it gets oxidised to form $ - CO{O^ - }$. So we understood that at position 1 and 3 at some point of time we will have $ - CO{O^ - }$ attached, hence option (a) is a possible product.
Also when benzene-1,3-dialdehyde gets reduced to form alcohols at position 1 and 3 we get $ - C{H_2}OH$. Hence option (b) is also a possible product.
And on the intermediate of this, it may also occur at some point of time one $ - CO{O^ - }$ and one $ - C{H_2}OH$ is formed. Therefore, option (c) is also a possible product.
Now, coming to option(d) we can see that the position of $ - CO{O^ - }$ and $ - C{H_2}OH$are at 1 and 4 respectively, which is never going to happen as the carbonyl group are present and 1 and 3 position of the compound. Hence this product never forms.
Hence, options A,B and C are correct.
Note:
In the presence of $\alpha $-hydrogen the reaction occurred is known as aldol condensation. In this reaction, two molecules of aldehydes or ketones having $\alpha $-hydrogen condense together in presence of a base like $NaOH$ to form $\beta $-hydroxy aldehyde or $\beta $-hydroxy ketone respectively which are collectively known as aldol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




