
Based on the group valency of elements write the molecular formula of the following complex following compounds giving justification for each:
(i) Oxide of first group elements.
(ii) Halides of the elements of group thirteen.
(iii) Compound formed when an element A of a group 2 combines with an element B of group seventeen.
Answer
565.8k+ views
Hint: We know that valence electrons are defined as the number of electrons present in the outermost shell and the number of electrons it accepts or donates to form a bond is called the valency of an electron.
For Example, The number of valence electrons in carbon is four and it needs four more electrons to have filled the outermost electron. Thus the valency of carbon is four.
Complete answer:
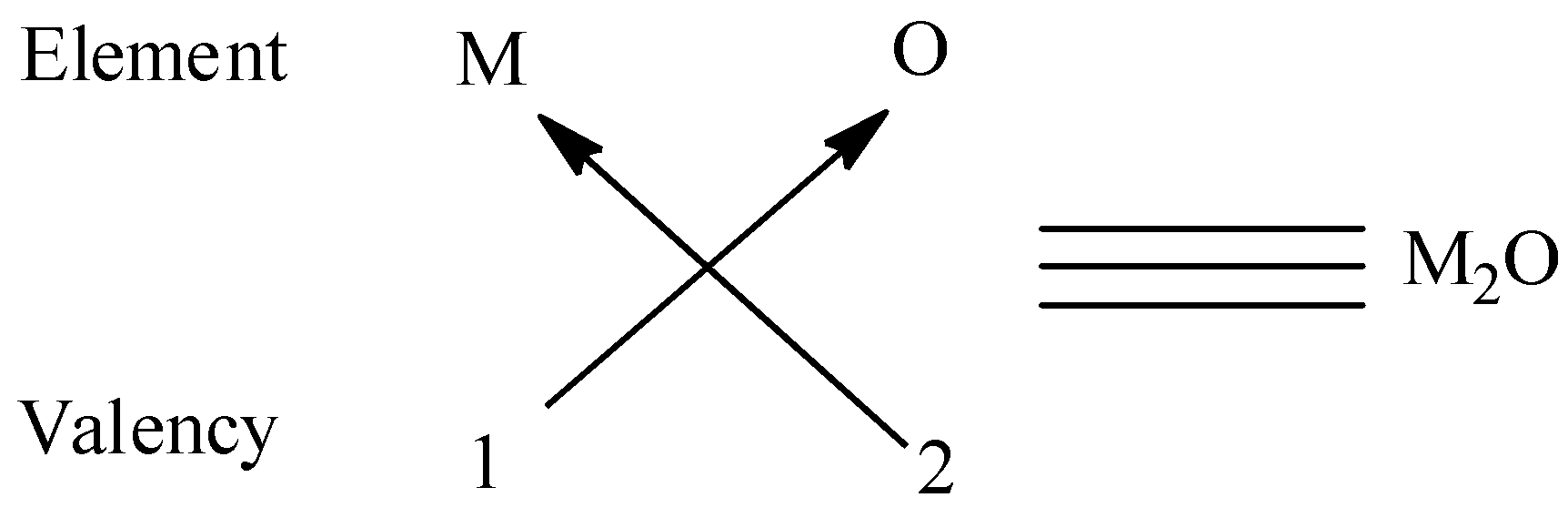
(i) We have to know that the valency of first group elements is \[1\] and the valency of oxygen is \[2\] and to satisfy the combining capacity of oxygen, \[2\] elements of first group are required. Thus the oxide of the first group elements has the common formula of \[{M_2}O\]. We can give an example for this type is $N{a_2}O$ .
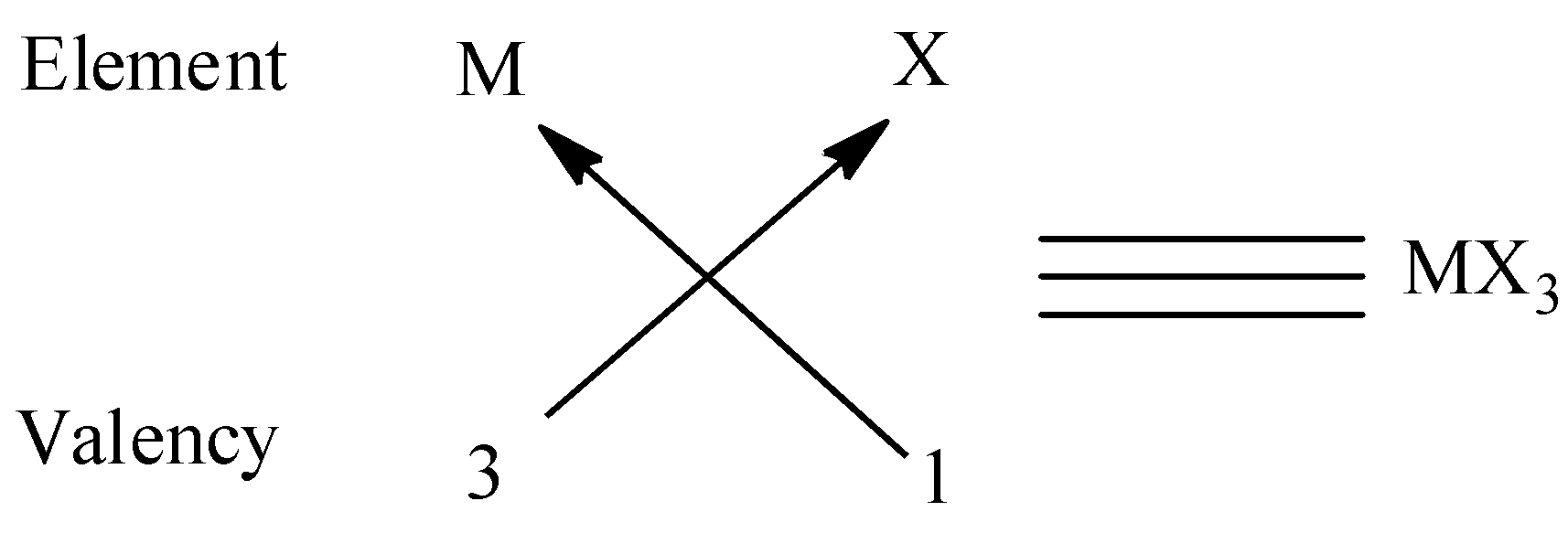
(ii) We know that the valency of group \[13\] elements is \[1\] and the valency of oxygen is\[2\] and to satisfy the combining capacity of group \[13\] elements, \[3\] halogens are required. Thus the molecular formula form is $M{X_3}$. We can give an example for this type is $BC{l_3}$ .
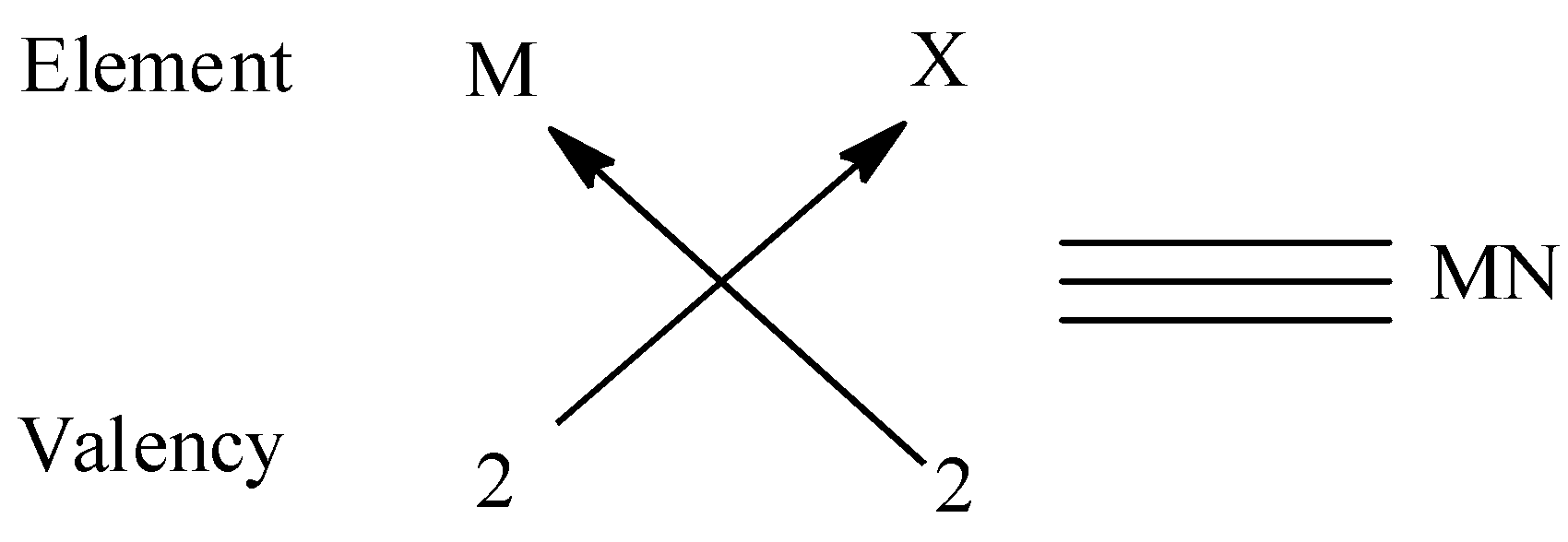
(iii) We must remember that the valency of group \[2\] elements is \[2\] and that of group \[17\] is \[1\] . Thus two elements of group \[17\] are required to combine with group 2 elements. Thus the molecular formula form is $MN$. We can give an example for this type is $MgO$.
Note:
We must remember that some elements vary in their capability to react with other elements, depending on the nature of the reaction; variable valence. For example, iron \[\left( {Fe} \right)\] may have a valence of both 2 & 3.
For Example, The number of valence electrons in carbon is four and it needs four more electrons to have filled the outermost electron. Thus the valency of carbon is four.
Complete answer:
(i) We have to know that the valency of first group elements is \[1\] and the valency of oxygen is \[2\] and to satisfy the combining capacity of oxygen, \[2\] elements of first group are required. Thus the oxide of the first group elements has the common formula of \[{M_2}O\]. We can give an example for this type is $N{a_2}O$ .

(ii) We know that the valency of group \[13\] elements is \[1\] and the valency of oxygen is\[2\] and to satisfy the combining capacity of group \[13\] elements, \[3\] halogens are required. Thus the molecular formula form is $M{X_3}$. We can give an example for this type is $BC{l_3}$ .

(iii) We must remember that the valency of group \[2\] elements is \[2\] and that of group \[17\] is \[1\] . Thus two elements of group \[17\] are required to combine with group 2 elements. Thus the molecular formula form is $MN$. We can give an example for this type is $MgO$.

Note:
We must remember that some elements vary in their capability to react with other elements, depending on the nature of the reaction; variable valence. For example, iron \[\left( {Fe} \right)\] may have a valence of both 2 & 3.
Recently Updated Pages
Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

The coating formed on the metals such as iron silver class 12 chemistry CBSE

Metals are refined by using different methods Which class 12 chemistry CBSE

What do you understand by denaturation of proteins class 12 chemistry CBSE

Assertion Nitrobenzene is used as a solvent in FriedelCrafts class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




