
Base catalysed aldol condensation occurs with:
A. benzaldehyde
B. \[2\]-methyl propanal
C. \[2,2\]-dimethyl propanal
D. Formaldehyde
Answer
583.8k+ views
Hint:The key step involves the abstraction of alpha hydrogen attached to the carbonyl carbon. The presence of this proton decides the fate of the reaction.
Complete step by step answer:
Aldol condensation is a carbon-carbon bond forming reaction in which a β-hydroxy ketone or β-hydroxy aldehyde is formed. The reaction initiated by formation of an enolate ion using a dilute base.
The enolate thus attacks a carbonyl carbon generating the β-hydroxy carbonyl compound. The mechanism of Aldol condensation is shown as:
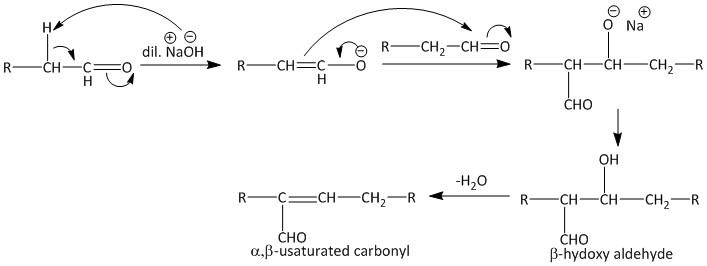
The dehydration of the β-hydroxy aldehyde ultimately leads to α,β-unsaturated aldehyde. The hydrogen present at the alpha position in the starting material is important. If no hydrogen is present at this position then the reaction will not initiate.
In order to find whether the Aldol condensation will occur or not on the given compounds we have to identify the structures.
Benzaldehyde:
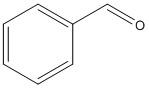 The structure of benzaldehyde does not have a alpha hydrogen atom and so will not undergo Aldol condensation.
The structure of benzaldehyde does not have a alpha hydrogen atom and so will not undergo Aldol condensation.
\[2\]-methyl propanal:
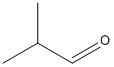 The structure of \[2\]-methyl propanal contains an alpha hydrogen atom. So the formation of enolate is possible for the given compound. Hence it will undergo Aldol condensation.
The structure of \[2\]-methyl propanal contains an alpha hydrogen atom. So the formation of enolate is possible for the given compound. Hence it will undergo Aldol condensation.
\[2,2\]-dimethyl propanal:
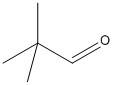 The structure of \[2,2\]-dimethyl propanal does not have an alpha hydrogen atom and so will not undergo Aldol condensation.
The structure of \[2,2\]-dimethyl propanal does not have an alpha hydrogen atom and so will not undergo Aldol condensation.
Formaldehyde:
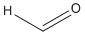 The structure formaldehyde does not have an alpha hydrogen atom and so will not undergo Aldol condensation.
The structure formaldehyde does not have an alpha hydrogen atom and so will not undergo Aldol condensation.
Thus option B is the correct answer, i.e. base catalysed aldol condensation occurs with \[2\]-methyl propanal.
Note:In contrast to Aldol condensation, another reaction also occurs in similar conditions. It is known as the Cannizzaro reaction. The absence of alpha hydrogen is important for this reaction to occur. The products formed in this reaction are a primary alcohol and a carboxylic acid.
Complete step by step answer:
Aldol condensation is a carbon-carbon bond forming reaction in which a β-hydroxy ketone or β-hydroxy aldehyde is formed. The reaction initiated by formation of an enolate ion using a dilute base.
The enolate thus attacks a carbonyl carbon generating the β-hydroxy carbonyl compound. The mechanism of Aldol condensation is shown as:

The dehydration of the β-hydroxy aldehyde ultimately leads to α,β-unsaturated aldehyde. The hydrogen present at the alpha position in the starting material is important. If no hydrogen is present at this position then the reaction will not initiate.
In order to find whether the Aldol condensation will occur or not on the given compounds we have to identify the structures.
Benzaldehyde:

\[2\]-methyl propanal:

\[2,2\]-dimethyl propanal:

Formaldehyde:

Thus option B is the correct answer, i.e. base catalysed aldol condensation occurs with \[2\]-methyl propanal.
Note:In contrast to Aldol condensation, another reaction also occurs in similar conditions. It is known as the Cannizzaro reaction. The absence of alpha hydrogen is important for this reaction to occur. The products formed in this reaction are a primary alcohol and a carboxylic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




