
Balance the following redox reactions:
${I_2}(s) + OC{l^ - }(aq) \to IO_3^ - (aq) + C{l^ - }(aq)$
(A) ${I_2}(s) + 5OC{l^ - }(aq) + {H_2}O(l) \to 2IO_3^ - (aq) + 5C{l^ - }(aq) + 2{H^ + }(aq)$
(B) ${I_2}(s) + 5OC{l^ - }(aq) + 2{H_2}O(l) \to IO_3^ - (aq) + 4C{l^ - }(aq) + 2{H^ + }(aq)$
(C) ${I_2}(s) + 3OC{l^ - }(aq) + 4{H_2}O(l) \to IO_3^ - (aq) + 4C{l^ - }(aq) + 2{H^ + }(aq)$
(D) ${I_2}(s) + 5OC{l^ - }(aq) + 7{H_2}O(l) \to IO_3^ - (aq) + 5C{l^ - }(aq) + 2{H^ + }(aq)$
Answer
585.3k+ views
Hint: Redox reaction is a reaction in which oxidation and reduction takes place simultaneously.
Oxidation is a process in which the oxidation number of atoms increases and Reduction is a process in which the oxidation number of atoms decreases.
Therefore, the basic principle of the reaction is that during redox reaction total increase in oxidation number must be equal to total decrease in oxidation number.
Complete step by step answer:
The chemical equation is:
${I_2}(s) + OC{l^ - }(aq) \to IO_3^ - (aq) + C{l^ - }(aq)$
Let us find the oxidation number of each element in the above reaction.
(i) Since ${I_2}(s)$ is present in an elemental state therefore its oxidation number is zero.
(ii) Oxidation number of $Cl$ in $OC{l^ - }:$ ${I_2}(s)$
-2 + x = - 1(because total charge on anion is -1)
x = - 1 + 2
x = + 1
(iii) Oxidation number of I in $IO_3^ - (aq):$
$\mathop I\limits^x \mathop {O_3^ - }\limits^{ - 2} = x + ( - 2 \times 3)$
x - 6 = - 1(because total charge on anion is -1)
x = - 1 + 6
x = + 5
(iv) Oxidation number of $C{l^ - }$ is - 1.
The complete redox reaction is given below:
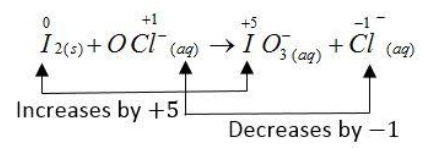
The oxidation number of the I atom increases from 0 to + 5 and the oxidation number of Cl atom decreases from + 1 to - 1.
${I_2}(s) + OC{l^ - }(aq) \to IO_3^ - (aq) + C{l^ - }(aq)$
Now balance the O-atom by adding a number of water molecules to the side which is deficient in O-atom.
${I_2}(s) + 5OC{l^ - }(aq) + {H_2}O(l) \to 2IO_3^ - (aq) + 5C{l^ - }(aq)$
Now balance the hydrogen atom by adding ${H^ + }$ to the deficient side.
${I_2}(s) + 5OC{l^ - }(aq) + {H_2}O(l) \to 2IO_3^ - (aq) + 5C{l^ - }(aq) + 2{H^ + }(aq)$
This is the balanced equation.
Therefore, from the above explanation the correct option is (A).
Note: Balancing of Hydrogen atoms depend upon the medium acidic or basic.
If reaction takes place in an acidic medium then ${H^ + }$ ion is added to the side deficient in Hydrogen atom.
If reaction takes place in the basic medium add ${H_2}O$ molecule to the H-atom deficient side and simultaneously add $O{H^ - }$ on the other side.
Oxidation is a process in which the oxidation number of atoms increases and Reduction is a process in which the oxidation number of atoms decreases.
Therefore, the basic principle of the reaction is that during redox reaction total increase in oxidation number must be equal to total decrease in oxidation number.
Complete step by step answer:
The chemical equation is:
${I_2}(s) + OC{l^ - }(aq) \to IO_3^ - (aq) + C{l^ - }(aq)$
Let us find the oxidation number of each element in the above reaction.
(i) Since ${I_2}(s)$ is present in an elemental state therefore its oxidation number is zero.
(ii) Oxidation number of $Cl$ in $OC{l^ - }:$ ${I_2}(s)$
-2 + x = - 1(because total charge on anion is -1)
x = - 1 + 2
x = + 1
(iii) Oxidation number of I in $IO_3^ - (aq):$
$\mathop I\limits^x \mathop {O_3^ - }\limits^{ - 2} = x + ( - 2 \times 3)$
x - 6 = - 1(because total charge on anion is -1)
x = - 1 + 6
x = + 5
(iv) Oxidation number of $C{l^ - }$ is - 1.
The complete redox reaction is given below:

The oxidation number of the I atom increases from 0 to + 5 and the oxidation number of Cl atom decreases from + 1 to - 1.
${I_2}(s) + OC{l^ - }(aq) \to IO_3^ - (aq) + C{l^ - }(aq)$
Now balance the O-atom by adding a number of water molecules to the side which is deficient in O-atom.
${I_2}(s) + 5OC{l^ - }(aq) + {H_2}O(l) \to 2IO_3^ - (aq) + 5C{l^ - }(aq)$
Now balance the hydrogen atom by adding ${H^ + }$ to the deficient side.
${I_2}(s) + 5OC{l^ - }(aq) + {H_2}O(l) \to 2IO_3^ - (aq) + 5C{l^ - }(aq) + 2{H^ + }(aq)$
This is the balanced equation.
Therefore, from the above explanation the correct option is (A).
Note: Balancing of Hydrogen atoms depend upon the medium acidic or basic.
If reaction takes place in an acidic medium then ${H^ + }$ ion is added to the side deficient in Hydrogen atom.
If reaction takes place in the basic medium add ${H_2}O$ molecule to the H-atom deficient side and simultaneously add $O{H^ - }$ on the other side.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




