
Bakelite is the first synthetic thermosetting polymer. (State whether true or false.)
(A) True
(B) False
Answer
570.9k+ views
Hint: Bakelite is a polymer which consists of cross-linked or heavily branched chain molecules which upon heating undergo extensive cross-linking in moulds and again become infusible. Polymer cannot be reused means it can’t regain its original shape after melting.
Complete answer:
Bakelite is the first thermosetting plastic made from synthetic components. It is a phenol formaldehyde resin formed from the condensation reaction of phenol with formaldehyde in the presence of a catalyst such as hydrochloric acid. The condensation product called Bakelite is soluble in alcohol or acetone. If the product is heated further the product becomes partially soluble and can still be softened but if heating is continued then there is a formation of insoluble hard gum. The resulting product is both infusible and insoluble as well.
We will be discussing about Bakelite which has two forms:
(A) Novolac: It is an intermediate formed when the concentration of phenol is less. Novolac is thermoplastic and has linear structure.
(B) Resol: The addition of formaldehyde again to novolac results in the formation of cross links between different chains. This cross linked structure is called Bakelite and is a thermosetting plastic because of the methyl bridges between the chains.
The structure of Bakelite is given below:
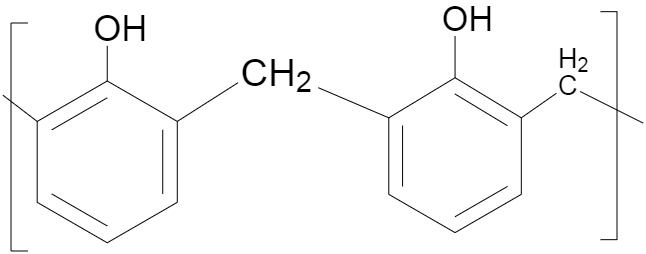
There are some useful properties of Bakelite which are known to us, namely,
(a) It can be quickly moulded.
(b) It is heat resistant and also resistant to electric current.
(c) It has high strength means it retains its form after extensive moulding.
Further, it has been established that Bakelite has been used for making the handles of utensils. So, we can say that Bakelite is one of the most important polymers used to make different objects.
Hence, option (A) is the correct answer.
Note: Bakelite is extensively used as adhesives and binding agents. It is used for protective purposes as well in the coating industry. The reaction that leads to the formation of Bakelite is condensation reaction and highly exothermic in nature. The loss of water molecules results in a cooling process. Also, the reaction should occur under pressure otherwise the product formed would be brittle.
Complete answer:
Bakelite is the first thermosetting plastic made from synthetic components. It is a phenol formaldehyde resin formed from the condensation reaction of phenol with formaldehyde in the presence of a catalyst such as hydrochloric acid. The condensation product called Bakelite is soluble in alcohol or acetone. If the product is heated further the product becomes partially soluble and can still be softened but if heating is continued then there is a formation of insoluble hard gum. The resulting product is both infusible and insoluble as well.
We will be discussing about Bakelite which has two forms:
(A) Novolac: It is an intermediate formed when the concentration of phenol is less. Novolac is thermoplastic and has linear structure.
(B) Resol: The addition of formaldehyde again to novolac results in the formation of cross links between different chains. This cross linked structure is called Bakelite and is a thermosetting plastic because of the methyl bridges between the chains.
The structure of Bakelite is given below:

There are some useful properties of Bakelite which are known to us, namely,
(a) It can be quickly moulded.
(b) It is heat resistant and also resistant to electric current.
(c) It has high strength means it retains its form after extensive moulding.
Further, it has been established that Bakelite has been used for making the handles of utensils. So, we can say that Bakelite is one of the most important polymers used to make different objects.
Hence, option (A) is the correct answer.
Note: Bakelite is extensively used as adhesives and binding agents. It is used for protective purposes as well in the coating industry. The reaction that leads to the formation of Bakelite is condensation reaction and highly exothermic in nature. The loss of water molecules results in a cooling process. Also, the reaction should occur under pressure otherwise the product formed would be brittle.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




