
What is the B product in the following reaction?
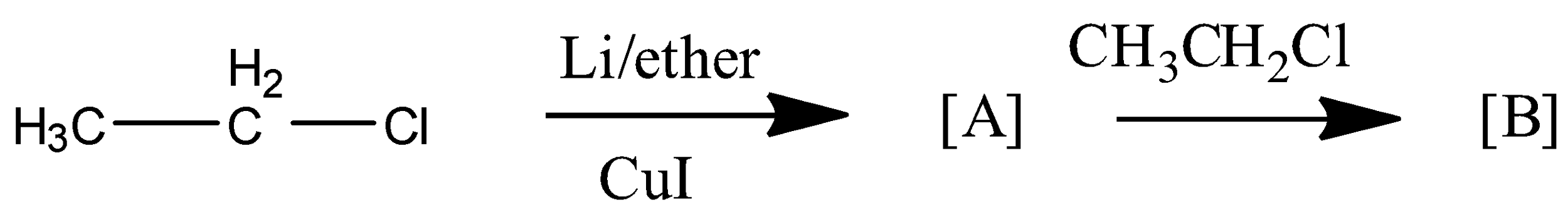

Answer
559.5k+ views
Hint: We will follow step to step formation of product. Li is electropositive in nature. The reaction here is organometallic reaction as metal copper is used here with organic compounds so the product formed will be organometallic.
Complete step by step answer:
In the first step a metal reacts with an organic compound. Organometallic reactions are a little different. Since metals are electropositive in nature and copper is divalent in nature it attaches two molecules of organic compound with it. This step is the formation of Gilman reagent.
In the second step it again reacts with another molecule of alkyl bromide which can be same or different and form alkane in the last i.e gilman reagent converts it into alkane.
The following is the mechanism
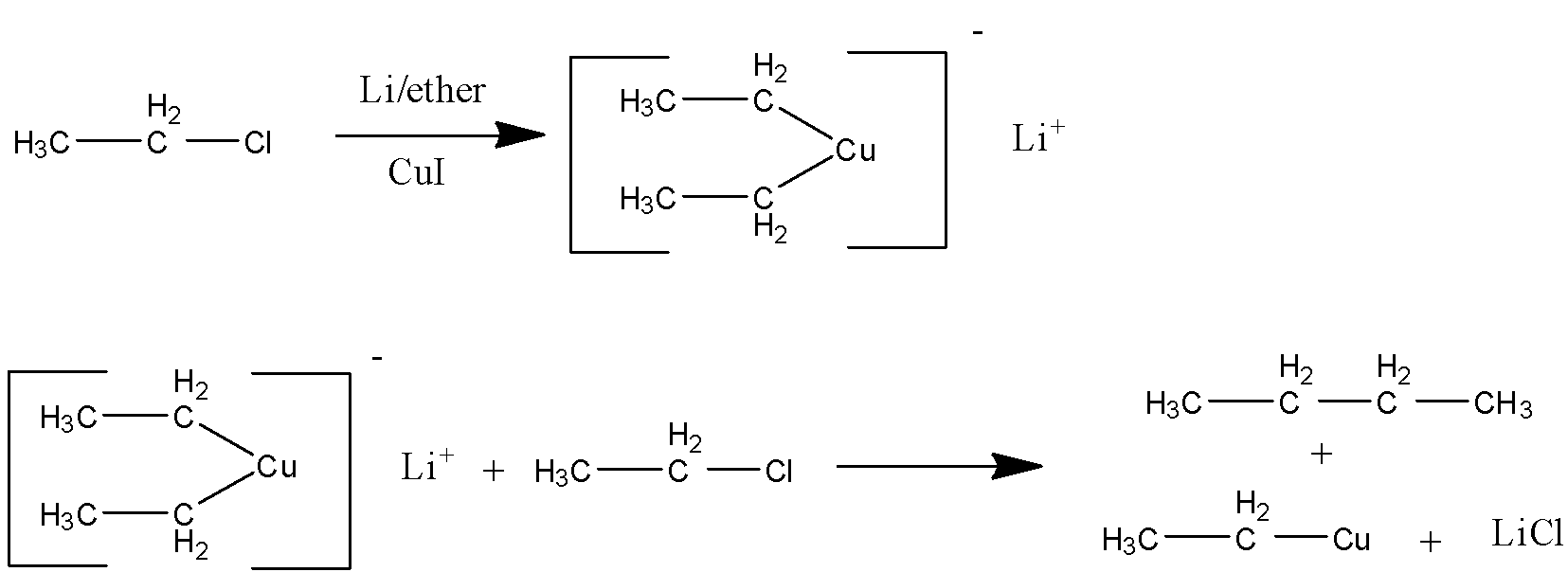
Here the product (B) is BUTANE
Gilman reagent is a di-organocopper compound which consists of lithium and copper. ${{\text{R}}_{\text{2}}}{\text{CuLi}}$ is the formula of gilman reagent here R is either aryl or alkyl group. This reagent is very important as it is different from Grignard reagent and organometallic reagent because it reacts with alkyl halides to replace the halide group with an alkyl group that can be same or different than the one inside gilman reagent.
Additional information: Organometallic reagents are reagents in which the metal is attached to organic carbon compounds. There are many organometallic reagents present, for example Grignard reagent, gilman reagent, dimethyl magnesium, organozinc compound etc.
Note:
Gilman reagent reacts with ${\alpha - \beta - \text{unsaturated ketone}}$ to form conjugate addition products which Grignard reagent or any other organometallic reagent cannot do. It is also a two step process just like this reaction.
Complete step by step answer:
In the first step a metal reacts with an organic compound. Organometallic reactions are a little different. Since metals are electropositive in nature and copper is divalent in nature it attaches two molecules of organic compound with it. This step is the formation of Gilman reagent.
In the second step it again reacts with another molecule of alkyl bromide which can be same or different and form alkane in the last i.e gilman reagent converts it into alkane.
The following is the mechanism

Here the product (B) is BUTANE
Gilman reagent is a di-organocopper compound which consists of lithium and copper. ${{\text{R}}_{\text{2}}}{\text{CuLi}}$ is the formula of gilman reagent here R is either aryl or alkyl group. This reagent is very important as it is different from Grignard reagent and organometallic reagent because it reacts with alkyl halides to replace the halide group with an alkyl group that can be same or different than the one inside gilman reagent.
Additional information: Organometallic reagents are reagents in which the metal is attached to organic carbon compounds. There are many organometallic reagents present, for example Grignard reagent, gilman reagent, dimethyl magnesium, organozinc compound etc.
Note:
Gilman reagent reacts with ${\alpha - \beta - \text{unsaturated ketone}}$ to form conjugate addition products which Grignard reagent or any other organometallic reagent cannot do. It is also a two step process just like this reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




