
B is
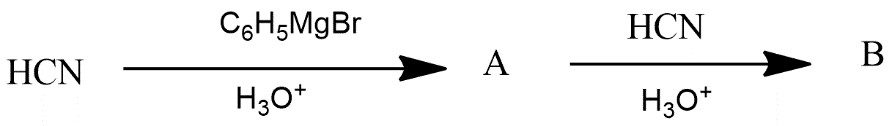
(A)
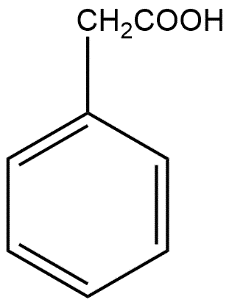
(B)
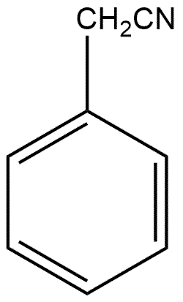
(C)
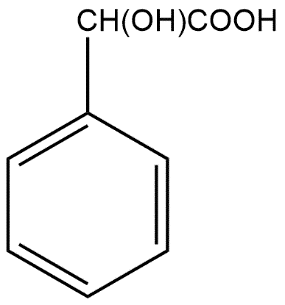
(D)
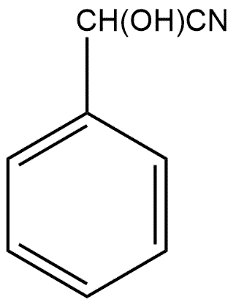





Answer
576.3k+ views
Hint: Grignard reagents are a type of organometallic chemistry which are very useful in organic chemistry. They exhibit strong nucleophilic qualities and also have the ability to form new carbon-carbon bonds.
Complete Step by step solution: A Grignard reagent is an organomagnesium compound which can be represented by the chemical formula R-Mg-X where R refers to an alkyl or aryl group and X refers to any halogen. These compounds are generally used for organic synthesis. The Grignard reaction is generally an organic reaction which is used to produce a number of products through the reaction of an organomagnesium compound which is also known as an electrophilic or Grignard reagent. The Grignard reagent is formed by the reaction of an alkyl or aryl halide with magnesium metal via a radical mechanism. For reactions of Grignard reagents it is necessary to ensure that no water is present because it would decompose the reagent readily. That’s why the majority of Grignard reactions occur in solvents such as anhydrous diethyl ether or tetrahydrofuran because the oxygen in these solvents stabilizes the magnesium reagent. In the given reaction first product i.e. A is given as:
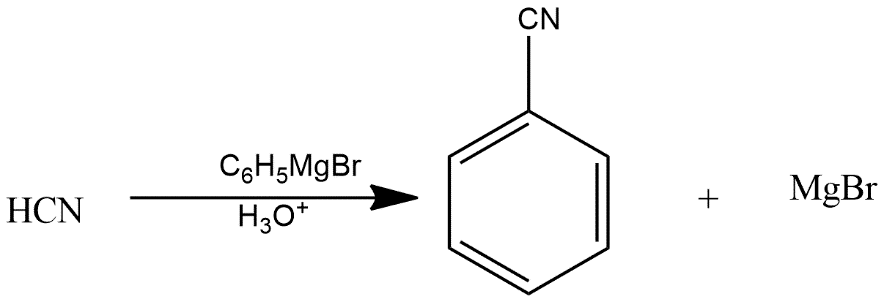
This forms benzene ring along with CN group which further reacts with \[{{H}_{3}}{{O}^{+}}\]to give the product B given as:
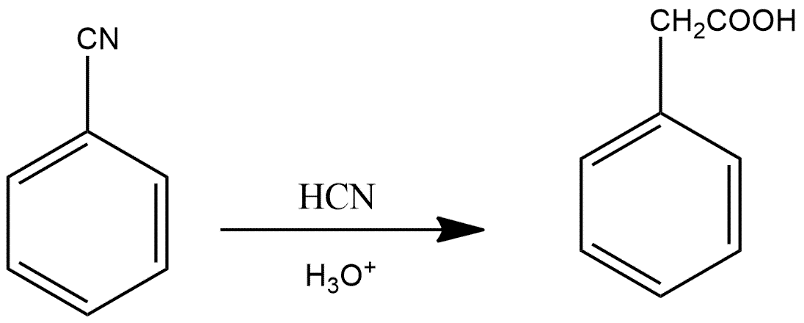
Thus we can say that option A is the correct answer.
Note: These reagents were discovered in 1912 by the French chemist Victor Grignard who won the Nobel Prize in Chemistry for these compounds. These are generally produced by reacting an aryl halide or an alkyl halide with magnesium.
Complete Step by step solution: A Grignard reagent is an organomagnesium compound which can be represented by the chemical formula R-Mg-X where R refers to an alkyl or aryl group and X refers to any halogen. These compounds are generally used for organic synthesis. The Grignard reaction is generally an organic reaction which is used to produce a number of products through the reaction of an organomagnesium compound which is also known as an electrophilic or Grignard reagent. The Grignard reagent is formed by the reaction of an alkyl or aryl halide with magnesium metal via a radical mechanism. For reactions of Grignard reagents it is necessary to ensure that no water is present because it would decompose the reagent readily. That’s why the majority of Grignard reactions occur in solvents such as anhydrous diethyl ether or tetrahydrofuran because the oxygen in these solvents stabilizes the magnesium reagent. In the given reaction first product i.e. A is given as:

This forms benzene ring along with CN group which further reacts with \[{{H}_{3}}{{O}^{+}}\]to give the product B given as:

Thus we can say that option A is the correct answer.
Note: These reagents were discovered in 1912 by the French chemist Victor Grignard who won the Nobel Prize in Chemistry for these compounds. These are generally produced by reacting an aryl halide or an alkyl halide with magnesium.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




