
What is B and the mechanism of reaction?
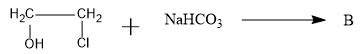
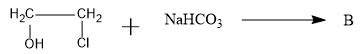
Answer
498k+ views
Hint: Alcohols are a group of organic compounds which have at least one hydroxyl group (-OH) and the hydrogen which is present in it is considered as a little acidic in nature. Now, sodium hydrogen carbonate is base and can abstract the acidic proton from the compound. So, this reaction will proceed further and en epoxide is formed.
Complete answer:
Alcohols are a group of organic compounds which have at least one hydroxyl group (-OH) and the hydrogen which is present in it is considered as a little acidic in nature.
Mechanism of the reaction is given as:
We know that sodium hydrogencarbonate with chemical formula $$NaHC{O_3}$$ is a base and can abstract the acidic proton from the compound. So the base will abstract the acidic proton as follows:
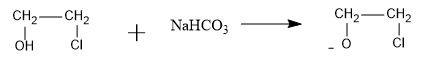
Now, the chloride ion is a very good leaving group, so it will leave the organic compound when negative charge of oxygen attacks the adjacent carbon and an epoxide is formed as follows:
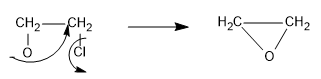
Hence, in this way the reaction mechanism proceeds.
So, the overall reaction is as follows:
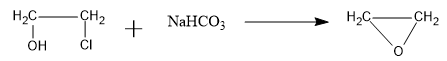
Therefore, the B compound is an epoxide:
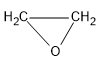
Note:
In these types of reactions in which a carbon adjacent to the alcoholic carbon has a leaving group and a base is added, first of all the base abstracts the acidic hydrogen of the alcoholic group and then negative charge on the oxygen attacks the adjacent carbon. So, the leaving group leaves the compound and forms an epoxide.
Complete answer:
Alcohols are a group of organic compounds which have at least one hydroxyl group (-OH) and the hydrogen which is present in it is considered as a little acidic in nature.
Mechanism of the reaction is given as:
We know that sodium hydrogencarbonate with chemical formula $$NaHC{O_3}$$ is a base and can abstract the acidic proton from the compound. So the base will abstract the acidic proton as follows:
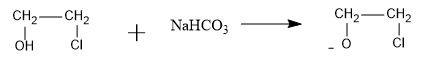
Now, the chloride ion is a very good leaving group, so it will leave the organic compound when negative charge of oxygen attacks the adjacent carbon and an epoxide is formed as follows:

Hence, in this way the reaction mechanism proceeds.
So, the overall reaction is as follows:
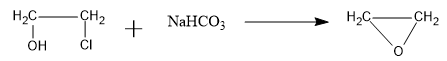
Therefore, the B compound is an epoxide:

Note:
In these types of reactions in which a carbon adjacent to the alcoholic carbon has a leaving group and a base is added, first of all the base abstracts the acidic hydrogen of the alcoholic group and then negative charge on the oxygen attacks the adjacent carbon. So, the leaving group leaves the compound and forms an epoxide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




