
What is the average oxidation number of sulfur in ${S_4}O_6^{2 - }$ ?
Answer
506.7k+ views
Hint: We have to know that, to address this inquiry, first we need to comprehend the significance of oxidation state. An oxidation is the cycle, which figures out which piece of the response is being oxidized and which part is being decreased in a redox response.
Complete answer:
As we realize that an oxidation state alludes to two things, they are given.
Oxidation just decreases as far as electron move happens in a redox response and electron-half-conditions. Presently, we should consider the construction of ${S_4}O_6^{2 - }$
The structure of ${S_4}O_6^{2 - }$ has to be drawn below,
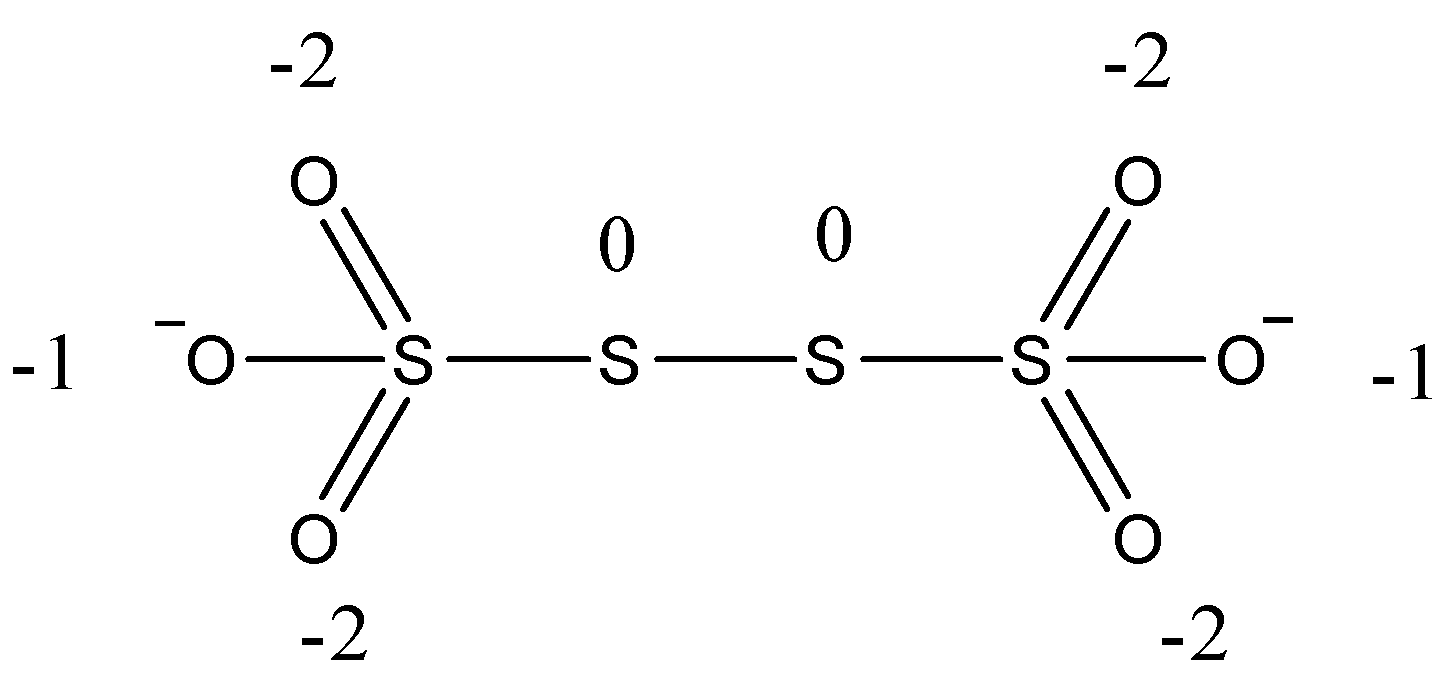
Then, at that point, we see that in the center two sulfur have zero oxidation states as an atom, which is fortified with comparative molecules, has an oxidation condition of nothing. Along these lines, the complete oxidation condition of sulfur in the compound is ten. Then, at that point, the oxidation condition of the furthest left and the furthest right sulfur is $ + 5$ , as oxygen is more electronegative. Subsequently, the oxidation condition of sulfur is given beneath,
$n - 2 - 2 - 1 + 0 = 0$
Therefore,
$n = 5$
Along these lines, the oxidation condition of sulfur is $ + 5$ detached $S - S$ linkage has zero oxidation state.
Subsequently, the oxidation state becomes $ + 5$ , $0$ , $0$ , $ + 5$ .
Note:
We need to recall that in oxoacids, sulfur shows a tetrahedral structure as for oxygen. Also, oxoacids are the acids that contain oxygen. The oxoacids have at least one $S = O$ bond and one $S - OH$ bond. Additionally, there are terminal peroxide gatherings, terminal $S = S$ , terminal and spanning oxygen particles in these oxoacids.
Complete answer:
As we realize that an oxidation state alludes to two things, they are given.
Oxidation just decreases as far as electron move happens in a redox response and electron-half-conditions. Presently, we should consider the construction of ${S_4}O_6^{2 - }$
The structure of ${S_4}O_6^{2 - }$ has to be drawn below,

Then, at that point, we see that in the center two sulfur have zero oxidation states as an atom, which is fortified with comparative molecules, has an oxidation condition of nothing. Along these lines, the complete oxidation condition of sulfur in the compound is ten. Then, at that point, the oxidation condition of the furthest left and the furthest right sulfur is $ + 5$ , as oxygen is more electronegative. Subsequently, the oxidation condition of sulfur is given beneath,
$n - 2 - 2 - 1 + 0 = 0$
Therefore,
$n = 5$
Along these lines, the oxidation condition of sulfur is $ + 5$ detached $S - S$ linkage has zero oxidation state.
Subsequently, the oxidation state becomes $ + 5$ , $0$ , $0$ , $ + 5$ .
Note:
We need to recall that in oxoacids, sulfur shows a tetrahedral structure as for oxygen. Also, oxoacids are the acids that contain oxygen. The oxoacids have at least one $S = O$ bond and one $S - OH$ bond. Additionally, there are terminal peroxide gatherings, terminal $S = S$ , terminal and spanning oxygen particles in these oxoacids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




