
At 530 K, glycerol reacts with oxalic acid to produce:
A) Allyl alcohol
B) Formic acid
C) Glyceraldehyde
D) Glycerol mono oxalate
Answer
566.7k+ views
Hint: The answer to this question is based on the concept of general organic reaction where heat is employed that is 530 K temperature in which an acid and polyols react together to form an alkenes or its alcohols. This fact gives the correct answer.
Complete Solution :
- In the classes of organic chemistry, we have studied the basic concepts that deal with the reaction mechanisms that include named reactions and also some of the basic reactions as well.
- We shall now see the reaction that takes place when glycerol is heated with oxalic acid at higher temperature.
- Glycerol is a polyol compound which is colourless and viscous liquid which is a non toxic and sweet tasting compound with the molecular formula ${{C}_{3}}{{H}_{8}}{{O}_{3}}$.
- On the other hand oxalic acid is and organic compound with the molecular formula ${{C}_{2}}{{H}_{2}}{{O}_{4}}$. It is white crystalline solid which forms a colourless solution with water.
Now, if glycerol is heated with oxalic acid at 530 K, glycerol will get converted into allyl alcohol as shown below:
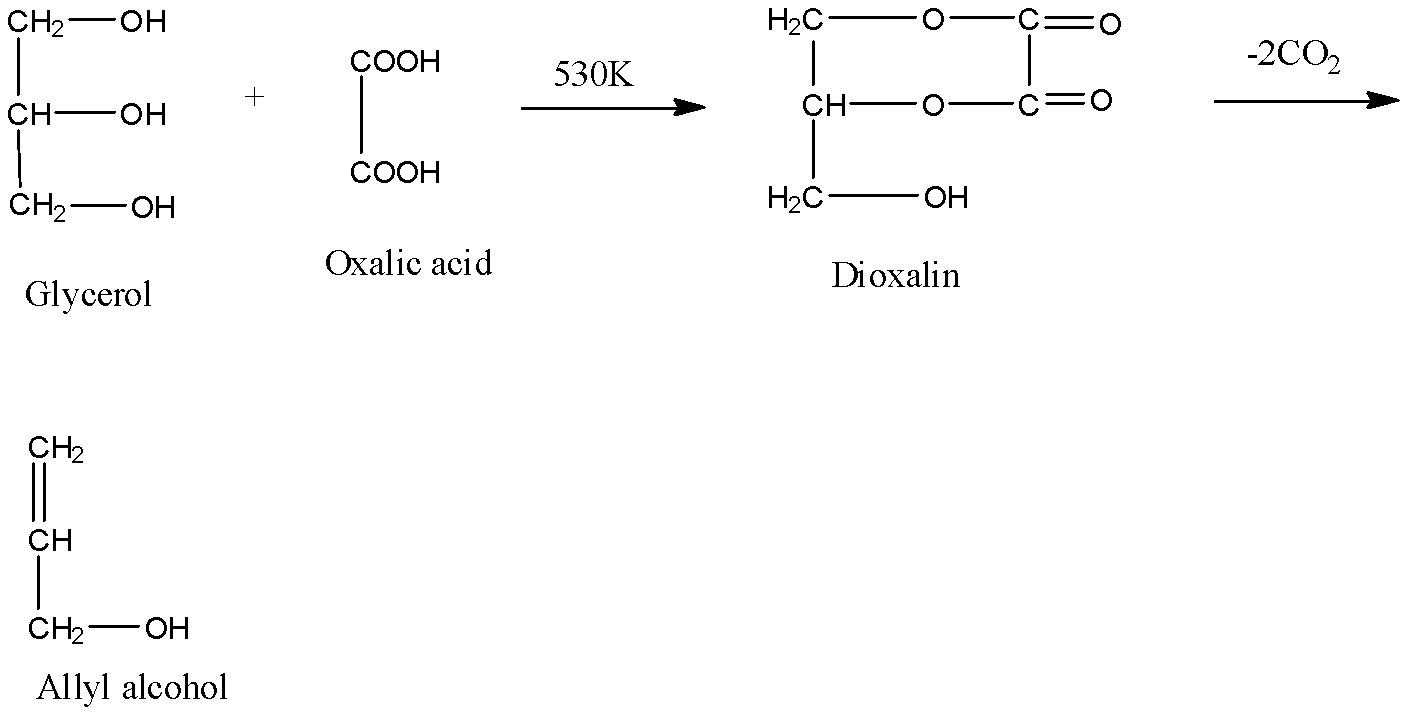
Here, we can see that the reaction goes via an intermediate compound formed that is dioxin. This high temperature gives allyl alcohol.
So, the correct answer is “Option A”.
Note: Note that glycerol is widely used for the treatment of wounds and burns as it has antiviral and antibacterial properties and also in the laboratories it comes out to be useful if you burn your hand with any strong acids accidentally.
Complete Solution :
- In the classes of organic chemistry, we have studied the basic concepts that deal with the reaction mechanisms that include named reactions and also some of the basic reactions as well.
- We shall now see the reaction that takes place when glycerol is heated with oxalic acid at higher temperature.
- Glycerol is a polyol compound which is colourless and viscous liquid which is a non toxic and sweet tasting compound with the molecular formula ${{C}_{3}}{{H}_{8}}{{O}_{3}}$.
- On the other hand oxalic acid is and organic compound with the molecular formula ${{C}_{2}}{{H}_{2}}{{O}_{4}}$. It is white crystalline solid which forms a colourless solution with water.
Now, if glycerol is heated with oxalic acid at 530 K, glycerol will get converted into allyl alcohol as shown below:

Here, we can see that the reaction goes via an intermediate compound formed that is dioxin. This high temperature gives allyl alcohol.
So, the correct answer is “Option A”.
Note: Note that glycerol is widely used for the treatment of wounds and burns as it has antiviral and antibacterial properties and also in the laboratories it comes out to be useful if you burn your hand with any strong acids accidentally.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




